Macula Densa Sensing and Signaling Mechanisms of Renin Release
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Ureter, Urinary Bladder & Urethra
Kidney, Ureter, Urinary bladder & Urethra Red: important. Black: in male|female slides. Gray: notes|extra. Editing file ➢ OBJECTIVES • The microscopic structure of the renal cortex and medulla. • The histology of renal corpuscle, proximal and distal tubules, loop of Henle, and collecting tubules & ducts. • The histological structure of juxtaglomerular apparatus. • The functional structures of the different parts of the kidney. • The microscopic structure of the Renal pelvis and ureter. • The microscopic structure of the urinary bladder and male and female urethra Histology team 437 | Renal block | All lectures ➢ KIDNEY o Cortex: Dark brown and granular. Content of cortex (renal corpuscle, PCT, loop of Henle, DCT, part of collecting tubule) o Medulla: 6-12 pyramid-shape regions (renal pyramids) content of medulla ( collecting duct, loop of Henle, collecting tubule) o The base of pyramid is toward the cortex (cortico-medullary border) o The apex (renal papilla) toward the hilum, it is perforated by 12 openings of the ducts of Bellini (Papillary “collecting” ducts) in region called area cribrosa. o The apex is surrounded by a minor calyx. o 3 or 4 minor calyces join to form 3 or 4 major calyces that form renal pelvis. o Pyramids are separated by cortical columns of Bertin (renal column) ➢ URINIFEROUS TUBULE o It is the functional unit of the kidney. o Is formed of: 1- Nephron. 2-Collecting tubule. o The tubules are densely packed. o The tubules are separated by thin stroma and basal lamina. Histology team 437 | Renal block | All lectures ➢ NEPHRON o There are 2 types of nephrons: a- Cortical nephrons. b- Juxtamedullary nephrons. -

Nitric Oxide Synthase in Macula Densa Regulates Glomerular Capillary
Proc. Nati. Acad. Sci. USA Vol. 89, pp. 11993-11997, December 1992 Pharmacology Nitric oxide synthase in macula densa regulates glomerular capillary pressure (kidney/tubuloglomerular feedback response/glomerular ifitration rate/afferent arteriole) CHRISTOPHER S. WILCOX*t, WILLIAM J. WELCH*, FERID MURADf, STEVEN S. GROSS§, GRAHAM TAYLOR¶, ROBERTO LEVI§, AND HARALD H. H. W. SCHMIDTII** *Division of Nephrology, Hypertension and Transplantation Departments of Medicine, Pharmacology and Therapeutics, University of Florida College of Medicine and Department of Veterans Affairs Medical Center, Gainesville, FL 32608; I'Department of Pharmacology, Northwestern University School of Medicine, Chicago, IL; tAbbott Laboratories, Abbott Park, IL 60064-3500; iDepartment of Pharmacology, Cornell University Medical College, New York, NY 10021; and IDepartment of Clinical Pharmacology, The Royal Postgraduate Medical School, Hammersmith Hospital, London, England W12 OHS Communicated by Robert F. Furchgott, September 3, 1992 ABSTRACT Tubular-fluid reabsorption by specialized Previous studies have established that L-arginine-derived cells of the nephron at the junction of the ascending limb of the nitric oxide (NO) is produced by several cells within the loop of Henle and the distal convoluted tubule, termed the kidney, including isolated glomerular mesangial (6) and en- macula densa, releases compounds causing vasoconstriction of dothelial cells (7), and a renal epithelial cell line (8), but its the adjacent afferent arteriole. Activation of this tubuloglo- integrative role in the control ofrenal function is not yet clear merular feedback response reduces glomerular capillary pres- (9). In the vessel wall, the endothelium can mediate vasodi- sure of the nephron and, hence, the glomerular filtration rate. lator responses to agents such as acetylcholine (10) and can The tubuloglomerular feedback response functions in a nega- blunt the actions of certain vasoconstrictors (11). -

Basic Histology (23 Questions): Oral Histology (16 Questions
Board Question Breakdown (Anatomic Sciences section) The Anatomic Sciences portion of part I of the Dental Board exams consists of 100 test items. They are broken up into the following distribution: Gross Anatomy (50 questions): Head - 28 questions broken down in this fashion: - Oral cavity - 6 questions - Extraoral structures - 12 questions - Osteology - 6 questions - TMJ and muscles of mastication - 4 questions Neck - 5 questions Upper Limb - 3 questions Thoracic cavity - 5 questions Abdominopelvic cavity - 2 questions Neuroanatomy (CNS, ANS +) - 7 questions Basic Histology (23 questions): Ultrastructure (cell organelles) - 4 questions Basic tissues - 4 questions Bone, cartilage & joints - 3 questions Lymphatic & circulatory systems - 3 questions Endocrine system - 2 questions Respiratory system - 1 question Gastrointestinal system - 3 questions Genitouirinary systems - (reproductive & urinary) 2 questions Integument - 1 question Oral Histology (16 questions): Tooth & supporting structures - 9 questions Soft oral tissues (including dentin) - 5 questions Temporomandibular joint - 2 questions Developmental Biology (11 questions): Osteogenesis (bone formation) - 2 questions Tooth development, eruption & movement - 4 questions General embryology - 2 questions 2 National Board Part 1: Review questions for histology/oral histology (Answers follow at the end) 1. Normally most of the circulating white blood cells are a. basophilic leukocytes b. monocytes c. lymphocytes d. eosinophilic leukocytes e. neutrophilic leukocytes 2. Blood platelets are products of a. osteoclasts b. basophils c. red blood cells d. plasma cells e. megakaryocytes 3. Bacteria are frequently ingested by a. neutrophilic leukocytes b. basophilic leukocytes c. mast cells d. small lymphocytes e. fibrocytes 4. It is believed that worn out red cells are normally destroyed in the spleen by a. neutrophils b. -

Renal Corpuscle Renal System > Histology > Histology
Renal Corpuscle Renal System > Histology > Histology Key Points: • The renal corpuscles lie within the renal cortex; • They comprise the glomerular, aka, Bowman's capsule and capillaries The capsule is a double-layer sac of epithelium: — The outer parietal layer folds upon itself to form the visceral layer. — The inner visceral layer envelops the glomerular capillaries. • As blood passes through the glomerular capillaries, aka, glomerulus, specific components, including water and wastes, are filtered to create ultrafiltrate. • The filtration barrier, which determines ultrafiltrate composition, comprises glomerular capillary endothelia, a basement membrane, and the visceral layer of the glomerular capsule. • Nephron tubules modify the ultrafiltrate to form urine. Overview Diagram: • Tuft of glomerular capillaries; blood enters the capillaries via the afferent arteriole, and exits via efferent arteriole. • The visceral layer of the glomerular capsule envelops the capillaries, then folds outwards to become the parietal layer. • The capsular space lies between the parietal and visceral layers; this space fills with ultrafiltrate. • Vascular pole = where the arterioles pass through the capsule • Urinary pole = where the nephron tubule begins • Distal tubule passes by the afferent arteriole. Details of Capillary and Visceral Layer: • Fenestrated glomerular capillary; fenestrations are small openings, aka, pores, in the endothelium that confer permeability. • Thick basement membrane overlies capillaries • Visceral layer comprises podocytes: — Cell bodies — Cytoplasmic extensions, called primary processes, give rise to secondary foot processes, aka, pedicles. • The pedicles interdigitate to form filtration slits; molecules pass through these slits to form the ultrafiltrate in the 1 / 3 capsular space. • Subpodocyte space; healthy podocytes do not adhere to the basement membrane. Clinical Correlation: • Podocyte injury causes dramatic changes in shape, and, therefore, their ability to filter substances from the blood. -
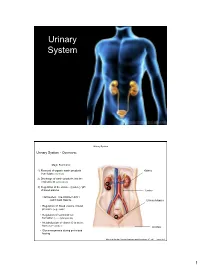
Urinary System
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -

Juxtaglomerular Apparatus Debajyoti Bhattacharya the Juxtaglomerular
Juxtaglomerular Apparatus Debajyoti Bhattacharya FNTA SEM II The juxtaglomerular apparatus (also known as the juxtaglomerular complex) is a structure in the kidney that regulates the function of each nephron, the functional units of the kidney. The juxtaglomerular apparatus is named because it is next to (juxta-[1]) the glomerulus. The juxtaglomerular apparatus is a specialized structure formed by the distal convoluted tubule and the glomerular afferent arteriole. It is located near the vascular pole of the glomerulus and its main function is to regulate blood pressure and the filtration rate of the glomerulus. The Macula densa is a collection of specialized epithelial cells in the distal convoluted tubule that detect sodium concentration of the fluid in the tubule. In response to elevated sodium, the macula densa cells trigger contraction of the afferent arteriole, reducing flow of blood to the glomerulus and the glomerular filtration rate. The juxtaglomerular cells, derived from smooth muscle cells, of the afferent arteriole secrete Renin when blood pressure in the arteriole falls. Renin increases blood pressure via the Renin-angiotensin-aldosterone system. Lacis cells, also called extraglomerular mesangial cells, are flat and elongated cells located near the macula densa. Their function remains unclear. The juxtaglomerular apparatus consists of three types of cells: 1. The macula densa, a part of the distal convoluted tubule of the same nephron 2. Juxtaglomerular cells, (also known as granular cells) which secrete Renin 3. Extraglomerular mesangial cells Structure: The juxtaglomerular apparatus comprises afferent and efferent arterioles, complemented by granular, Renin-secreting cells, the macula densa, a specialized group of distal tubular cells and Lacis cells (Goormaghtigh cells or Polkissen cells, polar cushion, extraglomerular mesangial cells). -

An in Vitro Approach to the Study of Macula Densa-Mediated Glomerular Hemodynamics
Kidney International, Vol. 38 (1990), pp. 1206—1210 TECHNICAL NOTE An in vitro approach to the study of macula densa-mediated glomerular hemodynamics SADAYOSHI ITO and OSCAR A. CARRETERO Hypertension Research Division, Heart and Vascular Institute, Hen,y Ford Hospital, Detroit, Michigan, USA Tubuloglomerular feedback (TGF), which operates between arm (500 U). The aorta was catheterized below the renal the tubule and the parent glomerulus, is important to renalarteries and clamped with a hemostat above the kidneys. The autoregulation and homeostasis of body fluid and electrolyteskidneys were perfused with cold medium 199 (Gibco Laborato- [1, 2]. Since the juxtaglomerular apparatus (JGA) displays anries, Grand Island, New York, USA) containing 5% bovine intimate anatomical relationship between the specialized tubu-serum albumin (BSA), then removed and sliced along the lar epithelial cells (macula densa) and the vasculature (afferentcorticomedullary axis. Slices were placed in ice-cold medium and efferent arterioles), it has long been suggested as the199 containing 5% BSA (medium 199-5% BSA) and microdis- anatomical site of TGF [3]. However, attempts to obtain directsected under a stereomicroscope (SZH; Olympus, Overland evidence to support this have been hindered by the anatomicalPark, Kansas, USA) at magnifications up to bOx, using thin complexity of the JGA. Since the JGA is located beneath thesteel needles and sharpened forceps (No. 5, Dumont; Fine tubular layer at some distance from the surface of the kidney,Science Tools, Inc., Belmont, California, USA). the macula densa is not accessible to direct micropuncture in An interlobular artery was removed from the remainder of vivo, nor is direct observation of the vascular pole possible. -

Il Sistema Genito-Urinario
Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema genito-urinario Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema urinario Functions The role of urinary system: • storing urine until a convenient time for disposal • providing the anatomical structures to transport this waste liquid to the outside of the body • cleansing the blood and ridding the body of wastes • regulation of pH • regulation of blood pressure • regulation of the concentration of solutes in the blood • regulation of the concentration of red blood cells by producing erythropoietin (EPO) in the kidney • perform the final synthesis step of vitamin D production in the kidney If the kidneys fail, these functions are compromised or lost altogether, with devastating effects on homeostasis. The affected individual might experience weakness, lethargy, shortness of breath, anemia, widespread edema (swelling), metabolic acidosis, rising potassium levels, heart arrhythmias, and more. Each of these functions is vital to your well- being and survival. Urine The urinary system’s ability to filter the blood resides in about 2 to 3 million tufts of specialized capillaries—the glomeruli—distributed more or less equally between the two kidneys. Because the glomeruli filter the blood based mostly on particle size, large elements like blood cells, platelets, antibodies, and albumen are excluded. The glomerulus is the first part of the nephron, which then continues as a highly specialized tubular structure responsible for creating the final urine composition. The glomeruli create about 200 liters of filtrate every day, yet you excrete less than two liters of waste you call urine. -

Possible Role of Adenosine in the Macula Densa Mechanism of Renin Release in Rabbits
Possible role of adenosine in the macula densa mechanism of renin release in rabbits. S Itoh, … , O A Carretero, R D Murray J Clin Invest. 1985;76(4):1412-1417. https://doi.org/10.1172/JCI112118. Research Article This study was designed to examine: (a) the effects of adenosine and its analogues on renin release in the absence of tubules, glomeruli, and macula densa, and (b) whether adenosine may be involved in a macula densa-mediated renin release mechanism. Rabbit afferent arterioles (Af) alone and afferent arterioles with macula densa attached (Af + MD) were microdissected and incubated for two consecutive 30-min periods. Hourly renin release rate from a single arteriole (or an arteriole with macula densa) was calculated and expressed as ng AI X h-1 X Af-1 (or Af + MD-1)/h (where AI is angiotensin I). Basal renin release rate from Af was 0.69 +/- 0.09 ng AI X h-1 X Af-1/h (means +/- SEM, n = 16) and remained stable for 60 min. Basal renin release rate from Af + MD was 0.20 +/- 0.04 ng AI X h-1 X Af + MD-1/h (n = 6), which was significantly lower (P less than 0.0025) than that from Af. When adenosine (0.1 microM) was added to Af, renin release decreased from 0.72 +/- 0.16 to 0.24 +/- 0.04 ng AI X h-1 X Af-1/h (P less than 0.025; n = 9). However, when adenosine was added to Af + MD, no significant change in renin release was observed. -

Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration During Hyperglycemia
BASIC RESEARCH www.jasn.org Macula Densa SGLT1-NOS1-Tubuloglomerular Feedback Pathway, a New Mechanism for Glomerular Hyperfiltration during Hyperglycemia Jie Zhang,1 Jin Wei,1 Shan Jiang,1 Lan Xu,2 Lei Wang,1 Feng Cheng,3 Jacentha Buggs,4 Hermann Koepsell,5 Volker Vallon,6 and Ruisheng Liu1 1Department of Molecular Pharmacology and Physiology, College of Medicine, 2Department of Biostatistics, College of Public Health, and 3Department of Pharmaceutical Science, College of Pharmacy, University of South Florida, Tampa, Florida; 4Advanced Organ Disease & Transplantation Institute, Tampa General Hospital, Tampa, Florida; 5Institute of Anatomy and Cell Biology, University of Würzburg, Würzburg, Germany; and 6Division of Nephrology and Hypertension, Department of Medicine, University of California, San Diego, La Jolla, California ABSTRACT Background Glomerular hyperfiltration is common in early diabetes and is considered a risk factor for later diabetic nephropathy. We propose that sodium-glucose cotransporter 1 (SGLT1) senses increases in luminal glucose at the macula densa, enhancing generation of neuronal nitric oxide synthase 1 (NOS1)– dependent nitric oxide (NO) in the macula densa and blunting the tubuloglomerular feedback (TGF) response, thereby promoting the rise in GFR. Methods We used microperfusion, micropuncture, and renal clearance of FITC–inulin to examine the effects of tubular glucose on NO generation at the macula densa, TGF, and GFR in wild-type and macula densa–specificNOS1knockoutmice. Results Acute intravenous injection of glucose induced hyperglycemia and glucosuria with increased GFR in mice. We found that tubular glucose blunts the TGF response in vivo and in vitro and stimulates NO generation at the macula densa. We also showed that SGLT1 is expressed at the macula densa; in the presence of tubular glucose, SGLT1 inhibits TGF and NO generation, but this action is blocked when the SGLT1 inhibitor KGA-2727 is present. -

The Morphology of the Juxtaglomerular Apparatus (JGA
Okajimas Folia Anat. Jpn., 63(6): 393-406, March 1987 Fine Structural Changes in the Three-Dimensional Structure of the Rat Juxtaglomerular Apparatus in Response to Water Deprivation By Sumie KIDOKORO Department of Anatomy, Yokohama City University School of Medicine, Kanazawaku, Yokohama, 236 Japan -Received for Publication, December 26, 1986- Key Words: juxtaglomerular apparatus, reconstruction, ultrastructure, water deprivation, rat Summary: Morphological changes in the juxtaglomerular apparatus (JGA) after water de- privation, especially those in the spatial relationships among the structural components of the JGA were investigated by electron microscopy of serial sections and the three-dimen- sional reconstruction. The most remarkable changes were observed after 1-day-water depriva- tion, i.e. the secretory granule-containing cell layer in the afferent arterioles was markedly increased in extent, and the ratio of contact area between the Goormaghtigh cells (GoCs) and the macula densa of the distal tubule to the whole surface of the GoC field was signifi- cantly reduced. A possible role of the GoCs in function of the JGA was discussed. The morphology of the juxtaglomerular a functional system for tubulo-glomerular apparatus (JGA) has been intensively studied feedback mechanism. It has been described by various approaches, including three- that the JGCs are also found in the efferent dimensional study using reconstruction arteriole as well as in the extraglomerular of serial sections (Barajas & Latta, '63; mesangial cells in some occasions. -

Nitric Oxide Synthesis in the Adult and Developing Kidney
Electrolyte & Blood Pressure 4:1-7, 2006 1 g1) Nitric Oxide Synthesis in the Adult and Developing Kidney Ki-Hwan Han1, Ju-Young Jung2, Ku-Yong Chung3, Hyang Kim4, and Jin Kim5 1Departments of Anatomy and 3Surgery, College of Medicine, Ewha Womans University, Seoul, Korea 2Department of Anatomy, College of Veterinary Medicine, Chungnam National University, Daejeon, Korea 4Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University, School of Medicine, Seoul, Korea 5Department of Anatomy and MRC for Cell Death Disease Research Center, College of Medicine, The Catholic University of Korea, Seoul, Korea Nitric oxide (NO) is synthesized within the adult and developing kidney and plays a critical role in the regulation of renal hemodynamics and tubule function. In the adult kidney, the regulation of NO synthesis is very cell type specific and subject to distinct control mechanisms of NO synthase (NOS) isoforms. Endothelial NOS (eNOS) is expressed in the endothelial cells of glomeruli, peritubular capillaries, and vascular bundles. Neuronal NOS (nNOS) is expressed in the tubular epithelial cells of the macula densa and inner medullary collecting duct. Furthermore, in the immature kidney, the expression of eNOS and nNOS shows unique patterns distinct from that is observed in the adult. This review will summarize the localization and presumable function of NOS isoforms in the adult and developing kidney. Key Words:Kidney, Development, Renal hemodynamics, Nitric oxide endothelial cells of almost all blood vessels except the Expression of NOS isoforms in the adult kidney venous system6, 7). The eNOS immunoreactivity is observed in the endothelial cells of glomeruli and peri- Nitric oxide (NO) is a lipophilic gas with unique tubular capillaries in the cortex, and in the endothelial physiological properties and plays an important role in cells of vascular bundles in the medulla.