Urinary System
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Kidney, Ureter, Urinary Bladder & Urethra
Kidney, Ureter, Urinary bladder & Urethra Red: important. Black: in male|female slides. Gray: notes|extra. Editing file ➢ OBJECTIVES • The microscopic structure of the renal cortex and medulla. • The histology of renal corpuscle, proximal and distal tubules, loop of Henle, and collecting tubules & ducts. • The histological structure of juxtaglomerular apparatus. • The functional structures of the different parts of the kidney. • The microscopic structure of the Renal pelvis and ureter. • The microscopic structure of the urinary bladder and male and female urethra Histology team 437 | Renal block | All lectures ➢ KIDNEY o Cortex: Dark brown and granular. Content of cortex (renal corpuscle, PCT, loop of Henle, DCT, part of collecting tubule) o Medulla: 6-12 pyramid-shape regions (renal pyramids) content of medulla ( collecting duct, loop of Henle, collecting tubule) o The base of pyramid is toward the cortex (cortico-medullary border) o The apex (renal papilla) toward the hilum, it is perforated by 12 openings of the ducts of Bellini (Papillary “collecting” ducts) in region called area cribrosa. o The apex is surrounded by a minor calyx. o 3 or 4 minor calyces join to form 3 or 4 major calyces that form renal pelvis. o Pyramids are separated by cortical columns of Bertin (renal column) ➢ URINIFEROUS TUBULE o It is the functional unit of the kidney. o Is formed of: 1- Nephron. 2-Collecting tubule. o The tubules are densely packed. o The tubules are separated by thin stroma and basal lamina. Histology team 437 | Renal block | All lectures ➢ NEPHRON o There are 2 types of nephrons: a- Cortical nephrons. b- Juxtamedullary nephrons. -
Urinary System
URINARY SYSTEM Ján Líška DVM, PhD Institut of Histology and Embryology, Faculty of Medicine, Comenius University Urinary system • The kidneys are the organ with multiple functions: • filtration of the blood • excretion of metabolic waste products and related removal of toxins • maintenance blood volume • regulation of acid-base balance • regulation of fluid and electrolyte balance • production of the hormones The other components of urinary system are accessory. Their function is essentially in order to eliminate urine. Urinary system - anatomy • Kidney are located in the retroperitoneal space • The surface of the kidney is covered by a fibrous capsule of dense connective tissue. • This capsule is coated with adipose capsule. • Each kidney is attached to a ureter, which carries urine to the bladder and urine is discharged out through the urethra. ANATOMIC STRUCTURE OF THE KIDNEY RENAL LOBES CORTEX outer shell columns Excretory portion medullary rays MEDULLA medullary pyramids HILUM Collecting system blood vessels lymph vessels major calyces nerves RENAL PELVIS minor calyces ureter Cortex is the outer layer surrounding the internal medulla. The cortex contains renal corpuscles, convoluted parts of prox. and dist. tubules. Renal column: the renal tissue projection between two medullary pyramids which supports the cortex. Renal pyramids: the conical segments within the medulla. They contain the ductal apparatus and stright parts of the tubules. They posses papilla - having openings through which urine passes into the calyces. Each pyramid together with the associated overlying cortex forms a renal lobe. renal pyramid papilla minor calix minor calyx Medullary rays: are in the middle of cortical part of the renal lobe, consisting of a group of the straight portiones of nephrons and the collec- medullary rays ting tubules (only straight tubules). -

Urinary System
OUTLINE 27.1 General Structure and Functions of the Urinary System 818 27.2 Kidneys 820 27 27.2a Gross and Sectional Anatomy of the Kidney 820 27.2b Blood Supply to the Kidney 821 27.2c Nephrons 824 27.2d How Tubular Fluid Becomes Urine 828 27.2e Juxtaglomerular Apparatus 828 Urinary 27.2f Innervation of the Kidney 828 27.3 Urinary Tract 829 27.3a Ureters 829 27.3b Urinary Bladder 830 System 27.3c Urethra 833 27.4 Aging and the Urinary System 834 27.5 Development of the Urinary System 835 27.5a Kidney and Ureter Development 835 27.5b Urinary Bladder and Urethra Development 835 MODULE 13: URINARY SYSTEM mck78097_ch27_817-841.indd 817 2/25/11 2:24 PM 818 Chapter Twenty-Seven Urinary System n the course of carrying out their specific functions, the cells Besides removing waste products from the bloodstream, the uri- I of all body systems produce waste products, and these waste nary system performs many other functions, including the following: products end up in the bloodstream. In this case, the bloodstream is ■ Storage of urine. Urine is produced continuously, but analogous to a river that supplies drinking water to a nearby town. it would be quite inconvenient if we were constantly The river water may become polluted with sediment, animal waste, excreting urine. The urinary bladder is an expandable, and motorboat fuel—but the town has a water treatment plant that muscular sac that can store as much as 1 liter of urine. removes these waste products and makes the water safe to drink. -
Ultra-Structure of Kidney the Excretory System Is Responsible for Filtering Wastes from the Blood and Both Forming and Secreting Urine
Ultra-Structure of kidney The excretory system is responsible for filtering wastes from the blood and both forming and secreting urine. These functions help to maintain the composition and volume of body fluids. Although it has far-reaching effects, the urinary system is relatively simple anatomically and consists of: Kidneys, Ureters, Bladder, and Urethra. The main organs are the kidneys, which filter blood and produce urine. The other parts are simply accessory structures for the transport and storage of urine. During the normal breakdown of protein and nucleic acids, nitrogen is released into the bloodstream. Some of this nitrogen is recycled to make new cellular products, but most of it is disposed of. The body has to have a way to rid itself of this unused nitrogen, as high levels in the blood can be toxic. Most of the nitrogen is bound with hydrogen as NH3 (ammonia), which is readily dissolved in water. For this reason, fish are able to excrete much of their nitrogen by simple diffusion into the surrounding water. Terrestrial animals have a different way of ridding their bodies of excess nitrogen. It is either excreted as uric acid or urea. Animals that are concerned about water loss, such as birds and reptiles, excrete the more concentrated uric acid as a pasty white material. Mammals, on the other hand, can excrete urea, along with more water. The mixture of urea, water, and other wastes is called 'urine.' Urine is still very concentrated in comparison to the blood, and the system that facilitates this concentration is the 'urinary system. -
Urinary System
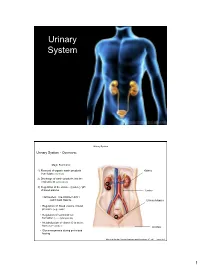
Urinary System Urinary System Urinary System - Overview: Major Functions: 1) Removal of organic waste products Kidney from fluids (excretion) 2) Discharge of waste products into the environment (elimination) 1 3) Regulation of the volume / [solute] / pH 3 of blood plasma Ureter HOWEVER, THE KIDNEY AIN’T JUST FOR PEE’IN… Urinary bladder • Regulation of blood volume / blood pressure (e.g., renin) • Regulation of red blood cell formation (i.e., erythropoietin) 2 • Metabolization of vitamin D to active form (Ca++ uptake) Urethra • Gluconeogenesis during prolonged fasting Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.1 1 Urinary System Renal ptosis: Kidneys drop to lower position due Functional Anatomy - Kidney: to loss of perirenal fat Located in the superior lumbar “Bar of soap” region 12 cm x 6 cm x 3 cm 150 g / kidney Layers of Supportive Tissue: Renal fascia: Peritoneal cavity Outer layer of dense fibrous connective tissue; anchors kidney in place Perirenal fat capsule: Fatty mass surrounding kidney; cushions kidney against blows Fibrous capsule: Transparent capsule on kidney; prevents infection of kidney from local tissues Kidneys are located retroperitoneal Marieb & Hoehn (Human Anatomy and Physiology, 8th ed.) – Figure 25.2 Urinary System Functional Anatomy - Kidney: Pyelonephritis: Inflammation of the kidney Pyramids appear striped due to parallel arrangement of capillaries / collecting tubes Renal cortex Renal medulla Renal pyramids Renal papilla Renal columns Renal hilum Renal pelvis • Entrance for blood vessels -
The Urinary System
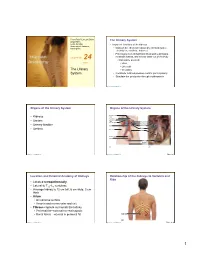
PowerPoint® Lecture Slides The Urinary System prepared by Leslie Hendon • Important functions of the kidneys University of Alabama, Birmingham • Maintain the chemical consistency of blood (water, electrolytes, acid/base balance) • Filter many liters of fluid from blood and send toxins, metabolic wastes, and excess water out of the body C H A P T E R 24 • Main waste products Part 1 • Urea • Uric acid The Urinary • Creatinine System • Contribute to blood pressure control (renin system) • Stimulate rbc production through erythropoietin Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Organs of the Urinary System Organs of the Urinary System Hepatic veins • Kidneys (cut) Esophagus (cut) Ureters Inferior vena • cava Renal artery Adrenal gland Renal hilum • Urinary bladder Aorta Renal vein Kidney • Urethra Iliac crest Ureter Rectum (cut) Uterus Urinary bladder Urethra (a) (b) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 24.1 Location and External Anatomy of Kidneys Relationship of the Kidneys to Vertebra and Ribs • Located retroperitoneally • Lateral to T12–L3 vertebrae • Average kidney is 12 cm tall, 6 cm wide, 3 cm thick • Hilum • On concave surface • Vessels and nerves enter and exit • Fibrous capsule surrounds the kidney • Perirenal fat—external to renal capsule • Renal fascia—external to perirenal fat 12th rib (b) Copyright © 2011 Pearson Education, Inc. Copyright © 2011 Pearson Education, Inc. Figure 24.2b 1 Position of the Kidneys with in the Posterior Internal -

Kidney Structure Renal Lobe Renal Lobule
Kidney Structure Capsule Hilum • ureter → renal pelvis → major and minor calyxes • renal artery and vein → segmental arteries → interlobar arteries → arcuate arteries → interlobular arteries Medulla • renal pyramids • cortical/renal columns Cortex • renal corpuscles • cortical labryinth of tubules • medullary rays Renal Lobe Renal Lobule = renal pyramid & overlying cortex = medullary ray & surrounding cortical labryinth Cortex Medulla Papilla Calyx Sobotta & Hammersen: Histology 1 Uriniferous Tubule Nephron + Collecting tubule Nephron Renal corpuscle produces glomerular ultrafiltrate from blood Ultrafiltrate is concentrated • Proximal tubule • convoluted • straight • Henle’s loop • thick descending • thin • thick ascending • Distal tubule • Collecting tubule Juxtaglomerular apparatus • macula densa in distal tubule •JG cells in afferent arteriole •extraglomerular mesangial cells Glomerulus • fenestrated capillaries • podocytes • intraglomerular mesangial cells 2 Urinary Filtration Urinary Membrane Membrane Podocytes • Endothelial cell • 70-90 nm fenestra restrict proteins > 70kd • Basal lamina • heparan sulfate is negatively charged • produced by endothelial cells & podocytes • phagocytosed by mesangial cells • Podocytes • pedicels 20-40 nm apart • diaphragm 6 nm thick with 3-5 nm slits • podocalyxin in glycocalyx is negatively charged 3 Juxtaglomerular Apparatus Macula densa in distal tubule • monitor Na+ content and volume in DT • low Na+: • stimulates JG cells to secrete renin • stimulates JG cells to dilate afferent arteriole • tall, -

(A) Adrenal Gland Inferior Vena Cava Iliac Crest Ureter Urinary Bladder
Hepatic veins (cut) Inferior vena cava Adrenal gland Renal artery Renal hilum Aorta Renal vein Kidney Iliac crest Ureter Rectum (cut) Uterus (part of female Urinary reproductive bladder system) Urethra (a) © 2018 Pearson Education, Inc. 1 12th rib (b) © 2018 Pearson Education, Inc. 2 Renal cortex Renal column Major calyx Minor calyx Renal pyramid (a) © 2018 Pearson Education, Inc. 3 Cortical radiate vein Cortical radiate artery Renal cortex Arcuate vein Arcuate artery Renal column Interlobar vein Interlobar artery Segmental arteries Renal vein Renal artery Minor calyx Renal pelvis Major calyx Renal Ureter pyramid Fibrous capsule (b) © 2018 Pearson Education, Inc. 4 Cortical nephron Fibrous capsule Renal cortex Collecting duct Renal medulla Renal Proximal Renal pelvis cortex convoluted tubule Glomerulus Juxtamedullary Ureter Distal convoluted tubule nephron Nephron loop Renal medulla (a) © 2018 Pearson Education, Inc. 5 Proximal convoluted Peritubular tubule (PCT) Glomerular capillaries capillaries Distal convoluted tubule Glomerular (DCT) (Bowman’s) capsule Efferent arteriole Afferent arteriole Cells of the juxtaglomerular apparatus Cortical radiate artery Arcuate artery Arcuate vein Cortical radiate vein Collecting duct Nephron loop (b) © 2018 Pearson Education, Inc. 6 Glomerular PCT capsular space Glomerular capillary covered by podocytes Efferent arteriole Afferent arteriole (c) © 2018 Pearson Education, Inc. 7 Filtration slits Podocyte cell body Foot processes (d) © 2018 Pearson Education, Inc. 8 Afferent arteriole Glomerular capillaries Efferent Cortical arteriole radiate artery Glomerular 1 capsule Three major renal processes: Rest of renal tubule 11 Glomerular filtration: Water and solutes containing smaller than proteins are forced through the filtrate capillary walls and pores of the glomerular capsule into the renal tubule. Peritubular 2 capillary 2 Tubular reabsorption: Water, glucose, amino acids, and needed ions are 3 transported out of the filtrate into the tubule cells and then enter the capillary blood. -

Il Sistema Genito-Urinario
Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema genito-urinario Ingegneria delle tecnologie per la salute Fondamenti di anatomia e istologia Il sistema urinario Functions The role of urinary system: • storing urine until a convenient time for disposal • providing the anatomical structures to transport this waste liquid to the outside of the body • cleansing the blood and ridding the body of wastes • regulation of pH • regulation of blood pressure • regulation of the concentration of solutes in the blood • regulation of the concentration of red blood cells by producing erythropoietin (EPO) in the kidney • perform the final synthesis step of vitamin D production in the kidney If the kidneys fail, these functions are compromised or lost altogether, with devastating effects on homeostasis. The affected individual might experience weakness, lethargy, shortness of breath, anemia, widespread edema (swelling), metabolic acidosis, rising potassium levels, heart arrhythmias, and more. Each of these functions is vital to your well- being and survival. Urine The urinary system’s ability to filter the blood resides in about 2 to 3 million tufts of specialized capillaries—the glomeruli—distributed more or less equally between the two kidneys. Because the glomeruli filter the blood based mostly on particle size, large elements like blood cells, platelets, antibodies, and albumen are excluded. The glomerulus is the first part of the nephron, which then continues as a highly specialized tubular structure responsible for creating the final urine composition. The glomeruli create about 200 liters of filtrate every day, yet you excrete less than two liters of waste you call urine. -

The Morphology of the Juxtaglomerular Apparatus (JGA
Okajimas Folia Anat. Jpn., 63(6): 393-406, March 1987 Fine Structural Changes in the Three-Dimensional Structure of the Rat Juxtaglomerular Apparatus in Response to Water Deprivation By Sumie KIDOKORO Department of Anatomy, Yokohama City University School of Medicine, Kanazawaku, Yokohama, 236 Japan -Received for Publication, December 26, 1986- Key Words: juxtaglomerular apparatus, reconstruction, ultrastructure, water deprivation, rat Summary: Morphological changes in the juxtaglomerular apparatus (JGA) after water de- privation, especially those in the spatial relationships among the structural components of the JGA were investigated by electron microscopy of serial sections and the three-dimen- sional reconstruction. The most remarkable changes were observed after 1-day-water depriva- tion, i.e. the secretory granule-containing cell layer in the afferent arterioles was markedly increased in extent, and the ratio of contact area between the Goormaghtigh cells (GoCs) and the macula densa of the distal tubule to the whole surface of the GoC field was signifi- cantly reduced. A possible role of the GoCs in function of the JGA was discussed. The morphology of the juxtaglomerular a functional system for tubulo-glomerular apparatus (JGA) has been intensively studied feedback mechanism. It has been described by various approaches, including three- that the JGCs are also found in the efferent dimensional study using reconstruction arteriole as well as in the extraglomerular of serial sections (Barajas & Latta, '63; mesangial cells in some occasions. -

The Distal Convoluted Tubule and Collecting Duct
Chapter 23 *Lecture PowerPoint The Urinary System *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • Urinary system rids the body of waste products. • The urinary system is closely associated with the reproductive system – Shared embryonic development and adult anatomical relationship – Collectively called the urogenital (UG) system 23-2 Functions of the Urinary System • Expected Learning Outcomes – Name and locate the organs of the urinary system. – List several functions of the kidneys in addition to urine formation. – Name the major nitrogenous wastes and identify their sources. – Define excretion and identify the systems that excrete wastes. 23-3 Functions of the Urinary System Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Diaphragm 11th and 12th ribs Adrenal gland Renal artery Renal vein Kidney Vertebra L2 Aorta Inferior vena cava Ureter Urinary bladder Urethra Figure 23.1a,b (a) Anterior view (b) Posterior view • Urinary system consists of six organs: two kidneys, two ureters, urinary bladder, and urethra 23-4 Functions of the Kidneys • Filters blood plasma, separates waste from useful chemicals, returns useful substances to blood, eliminates wastes • Regulate blood volume and pressure by eliminating or conserving water • Regulate the osmolarity of the body fluids by controlling the relative amounts of water and solutes -

Renal Ultrasound (Basic Principles) BMUS Study Day
Renal Ultrasound (Basic Principles) BMUS Study Day Rosie Conlon Clinical Specialist Sonographer NATIONAL REHABILITATION HOSPITAL Dun laoghaire, Dublin Sat 15th October 2016 WHY? “Bones can break, muscles can atrophy, glands can loaf about and even the brain can sleep without immediate danger to survival. BUT when the kidneys fail…. Neither bone, muscle gland nor brain could carry on.” Homer William Smith, “The Evolution of the Kidney”, Lectures on the Kidney (1943). Renal Ultrasound (Basic Principles) and BMUS Study Case PREPARATION • 500mls 1 hour before, avoiding • Operator Dependant micturition. • Catheter clamped 1.5 hours • Real Time before. • Fluids by PEG 1.5 hours before. • Reproducible • Non-invasive • Inspiration WHAT? - PROTOCOL • Both Kidneys • Urinary Bladder • +/- Residual Volume • Pelvic Surveillance • Aorta • Local protocol pertinent to the population • Full bladder The most important step in diagnosis is realising that it might exist. Renal Ultrasound (Basic Principles) and BMUS Study Case RIGHT KIDNEY-TECHNIQUE • A 3.5-5 MHz probe is typically used to scan the kidney. For the right kidney, have the patient lie supine and place the probe in the right lower intercostal space in the midaxillary line. Use the liver as your “acoustic window” and aim the probe slightly posteriorly (toward the kidney). Gently rock the probe (up and down or side to side) to scan the entire kidney. If needed, you can have the patient inspire or exhale, which allows for subtle movement of the kidney. • Obtain longitudinal (long axis) and transverse (short axis) views. Renal Ultrasound (Basic Principles) and BMUS Study Case ANATOMY Hepatic Veins Spleen Celiac axis Liver SMA Left Right Renal artery kidney kidney Renal vein Renal Ultrasound (Basic Principles) and BMUS Study Case NORMAL Renal Ultrasound (Basic Principles) and BMUS Study Case LEFT KIDNEY-TECHNIQUE • For the left kidney have the patient lie supine or in the right lateral decubitus position.