(Nitags) and WHO Immunisation-Related Advisory Committees
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

(ACIP) General Best Guidance for Immunization
9. Special Situations Updates Major revisions to this section of the best practices guidance include the timing of intramuscular administration and the timing of clotting factor deficiency replacement. Concurrent Administration of Antimicrobial Agents and Vaccines With a few exceptions, use of an antimicrobial agent does not interfere with the effectiveness of vaccination. Antibacterial agents have no effect on inactivated, recombinant subunit, or polysaccharide vaccines or toxoids. They also have no effect on response to live, attenuated vaccines, except BCG vaccines. Antimicrobial or immunosuppressive agents may interfere with the immune response to BCG and should only be used under medical supervision (for additional information, see www.merck.com/product/usa/pi_circulars/b/bcg/bcg_pi.pdf). Antiviral drugs used for treatment or prophylaxis of influenza virus infections have no effect on the response to inactivated influenza vaccine (2). However, live, attenuated influenza vaccine should not be administered until 48 hours after cessation of therapy with antiviral influenza drugs. If feasible, to avoid possible reduction in vaccine effectiveness, antiviral medication should not be administered for 14 days after LAIV administration (2). If influenza antiviral medications are administered within 2 weeks after receipt of LAIV, the LAIV dose should be repeated 48 or more hours after the last dose of zanamavir or oseltamivir. The LAIV dose should be repeated 5 days after peramivir and 17 days after baloxavir. Alternatively, persons receiving antiviral drugs within the period 2 days before to 14 days after vaccination with LAIV may be revaccinated with another approved vaccine formulation (e.g., IIV or recombinant influenza vaccine). Antiviral drugs active against herpesviruses (e.g., acyclovir or valacyclovir) might reduce the efficacy of vaccines containing live, attenuated varicella zoster virus (i.e., Varivax and ProQuad) (3,4). -

ALTIMMUNE, INC. (Exact Name of Registrant As Specified in Its Charter)
UNITED STATES SECURITIES AND EXCHANGE COMMISSION Washington, D.C. 20549 FORM 10-K (Mark One) ☒ Annual Report Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934 For the fiscal year ended December 31, 2019 ☐ Transition Report under Section 13 or 15(d) of the Securities Exchange Act of 1934 For the transition period from to Commission File Number: 001-32587 ALTIMMUNE, INC. (Exact name of registrant as specified in its charter) Delaware 20-2726770 (State or other jurisdiction of (I.R.S. Employer incorporation or organization) Identification No.) 910 Clopper Road, Suite 201S, Gaithersburg, MD 20878 (Address of principal executive offices) (Zip Code) Registrant’s telephone number, including area code (240) 654-1450 Securities registered pursuant to Section 12(b) of the Act: Title of each class Trading Symbol(s) Name of each exchange on which registered Common stock, par value $0.0001 per share ALT The NASDAQ Global Market Securities registered pursuant to Section 12(g) of the Act: None Indicate by check mark if the registrant is a well-known seasoned issuer, as defined in Rule 405 of the Securities Act. Yes ☐ No ☒ Indicate by check mark if the registrant is not required to file reports pursuant to Section 13 or 15(d) of the Act. Yes ☐ No ☒ Indicate by check mark whether the registrant (1) has filed all reports required to be filed by Section 13 or 15(d) of the Securities Exchange Act of 1934 during the preceding 12 months (or for such shorter period that the registrant was required to file such reports), and (2) has been subject to such filing requirements for the past 90 days. -

Recommended Adult Immunization Schedule
Recommended Adult Immunization Schedule UNITED STATES for ages 19 years or older 2021 Recommended by the Advisory Committee on Immunization Practices How to use the adult immunization schedule (www.cdc.gov/vaccines/acip) and approved by the Centers for Disease Determine recommended Assess need for additional Review vaccine types, Control and Prevention (www.cdc.gov), American College of Physicians 1 vaccinations by age 2 recommended vaccinations 3 frequencies, and intervals (www.acponline.org), American Academy of Family Physicians (www.aafp. (Table 1) by medical condition and and considerations for org), American College of Obstetricians and Gynecologists (www.acog.org), other indications (Table 2) special situations (Notes) American College of Nurse-Midwives (www.midwife.org), and American Academy of Physician Assistants (www.aapa.org). Vaccines in the Adult Immunization Schedule* Report y Vaccines Abbreviations Trade names Suspected cases of reportable vaccine-preventable diseases or outbreaks to the local or state health department Haemophilus influenzae type b vaccine Hib ActHIB® y Clinically significant postvaccination reactions to the Vaccine Adverse Event Hiberix® Reporting System at www.vaers.hhs.gov or 800-822-7967 PedvaxHIB® Hepatitis A vaccine HepA Havrix® Injury claims Vaqta® All vaccines included in the adult immunization schedule except pneumococcal 23-valent polysaccharide (PPSV23) and zoster (RZV) vaccines are covered by the Hepatitis A and hepatitis B vaccine HepA-HepB Twinrix® Vaccine Injury Compensation Program. Information on how to file a vaccine injury Hepatitis B vaccine HepB Engerix-B® claim is available at www.hrsa.gov/vaccinecompensation. Recombivax HB® Heplisav-B® Questions or comments Contact www.cdc.gov/cdc-info or 800-CDC-INFO (800-232-4636), in English or Human papillomavirus vaccine HPV Gardasil 9® Spanish, 8 a.m.–8 p.m. -

Immune Effector Mechanisms and Designer Vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA
EXPERT REVIEW OF VACCINES https://doi.org/10.1080/14760584.2019.1674144 REVIEW How vaccines work: immune effector mechanisms and designer vaccines Stewart Sell Wadsworth Center, New York State Department of Health, Empire State Plaza, Albany, NY, USA ABSTRACT ARTICLE HISTORY Introduction: Three major advances have led to increase in length and quality of human life: Received 6 June 2019 increased food production, improved sanitation and induction of specific adaptive immune Accepted 25 September 2019 responses to infectious agents (vaccination). Which has had the most impact is subject to debate. KEYWORDS The number and variety of infections agents and the mechanisms that they have evolved to allow Vaccines; immune effector them to colonize humans remained mysterious and confusing until the last 50 years. Since then mechanisms; toxin science has developed complex and largely successful ways to immunize against many of these neutralization; receptor infections. blockade; anaphylactic Areas covered: Six specific immune defense mechanisms have been identified. neutralization, cytolytic, reactions; antibody- immune complex, anaphylactic, T-cytotoxicity, and delayed hypersensitivity. The role of each of these mediated cytolysis; immune immune effector mechanisms in immune responses induced by vaccination against specific infectious complex reactions; T-cell- mediated cytotoxicity; agents is the subject of this review. delayed hypersensitivity Expertopinion: In the past development of specific vaccines for infections agents was largely by trial and error. With an understanding of the natural history of an infection and the effective immune response to it, one can select the method of vaccination that will elicit the appropriate immune effector mechanisms (designer vaccines). These may act to prevent infection (prevention) or eliminate an established on ongoing infection (therapeutic). -

Varicella (Chickenpox): Questions and Answers Q&A Information About the Disease and Vaccines
Varicella (Chickenpox): Questions and Answers Q&A information about the disease and vaccines What causes chickenpox? more common in infants, adults, and people with Chickenpox is caused by a virus, the varicella-zoster weakened immune systems. virus. How do I know if my child has chickenpox? How does chickenpox spread? Usually chickenpox can be diagnosed by disease his- Chickenpox spreads from person to person by direct tory and appearance alone. Adults who need to contact or through the air by coughing or sneezing. know if they’ve had chickenpox in the past can have It is highly contagious. It can also be spread through this determined by a laboratory test. Chickenpox is direct contact with the fluid from a blister of a per- much less common now than it was before a vaccine son infected with chickenpox, or from direct contact became available, so parents, doctors, and nurses with a sore from a person with shingles. are less familiar with it. It may be necessary to perform laboratory testing for children to confirm chickenpox. How long does it take to show signs of chickenpox after being exposed? How long is a person with chickenpox contagious? It takes from 10 to 21 days to develop symptoms after Patients with chickenpox are contagious for 1–2 days being exposed to a person infected with chickenpox. before the rash appears and continue to be conta- The usual time period is 14–16 days. gious through the first 4–5 days or until all the blisters are crusted over. What are the symptoms of chickenpox? Is there a treatment for chickenpox? The most common symptoms of chickenpox are rash, fever, coughing, fussiness, headache, and loss of appe- Most cases of chickenpox in otherwise healthy children tite. -

Trends in Vaccine Availability and Novel Vaccine Delivery Technologies: 2008–2025
Landscape Analysis Trends in vaccine availability and novel vaccine delivery technologies: 2008–2025 July 2008 Bâtiment Avant Centre Phone: 33.450.28.00.49 13 Chemin du Levant Fax: 33.450.28.04.07 01210 Ferney Voltaire www.path.org France www.who.int Landscape Analysis Trends in vaccine availability and novel vaccine delivery technologies: 2008–2025 July 1, 2008 Version: January 22, 2009 ii Table of contents Acronyms and abbreviations.......................................................................................................................................... iv Executive summary......................................................................................................................................................... 1 TRENDS IN VACCINE AVAILABILITY: 2008–2025 ............................................................................................. 2 Choice of vaccine types to be surveyed........................................................................................................................... 2 Vaccine availability and use: 2008–2025........................................................................................................................ 2 Vaccine availability............................................................................................................................................... 2 Delivery strategies................................................................................................................................................. 4 Extensions -

Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures
Dissertation Dissemination of Vaccine Misinformation on Twitter and Its Countermeasures Christine Chen This document was submitted as a dissertation in March 2021 in partial fulfillment of the requirements of the doctoral degree in public policy analysis at the Pardee RAND Graduate School. The faculty committee that supervised and approved the dissertation consisted of Luke Matthews (Chair), Sarah Nowak and Jeremy Miles. The external reader was Jennifer Golbeck. This dissertation was generously supported by the Anne and James Rothenberg Dissertation Award. PARDEE RAND GRADUATE SCHOOL For more information on this publication, visit http://www.rand.org/pubs/rgs_dissertations/RGSDA1332-1.html Published 2021 by the RAND Corporation, Santa Monica, Calif. is a registered trademarK Limited Print and Electronic Distribution Rights This document and trademarK(s) contained herein are protected by law. This representation of RAND intellectual property is provided for noncommercial use only. Unauthorized posting of this publication online is prohibited. Permission is given to duplicate this document for personal use only, as long as it is unaltered and complete. Permission is reQuired from RAND to reproduce, or reuse in another form, any of its research documents for commercial use. For information on reprint and linking permissions, please visit www.rand.org/pubs/permissions.html. The RAND Corporation is a research organization that develops solutions to public policy challenges to help maKe communities throughout the world safer and more secure, healthier and more prosperous. RAND is nonprofit, nonpartisan, and committed to the public interest. RAND’s publications do not necessarily reflect the opinions of its research clients and sponsors. Support RAND MaKe a tax-deductible charitable contribution at www.rand.org/giving/contribute www.rand.org Abstract Outbreaks of vaccine preventable diseases have continued to affect many parts of the United States. -

The Study of Thimerosal and Autism
The Study of Thimerosal and Autism Documentation and Codebook for the Child Vaccination Histories File: Cambridge, MA Lexington, MA Hadley, MA Bethesda, MD September 3, 2010 Chicago, IL Prepared for Frank DeStefano National Immunization Program Centers for Disease Control and Prevention Atlanta, GA 30329 Prepared by Cristofer Price Yeqin He Abt Associates Inc. Suite 800 North 4550 Montgomery Avenue Bethesda, MD 20814-3343 This documentation was prepared by Cristofer Price and Yeqin He of Abt Associates Inc. for the Immunization Safety Office (ISO) of the Centers for Disease Control and Prevention (CDC) Atlanta, GA 30333. Questions about the documentation, substantive questions, or technical issues regarding the data file should be directed to the CDC ISO, MS D26, 1600 Clifton Road, Atlanta, Georgia 30333 (404-639-8256). Suggested Citation for Study of Thimerosal and Autism Public Use Data Set: Price, C.S., He, Y. (2010) The Study of Thimerosal and Autism: Data Set Documentation. Bethesda MD: Abt Associates, Inc; Prepared for the National Immunization Program Centers for Disease Control and Prevention Atlanta, GA Abt Associates Inc. 1 Documentation and Codebook for the Child Vaccination Histories File 1. Introduction to the Child Vaccination Histories File............................................... 2 2. File Formats and Variable Descriptions................................................................... 4 1. Introduction to the Child Vaccination Histories File The Child Vaccination Histories File contains the data that were used to construct the measures of early childhood (postnatal) exposure to ethylmercury from thimerosal- containing vaccines and immune globulin preparations received by the study children during the age range spanning birth to 20 months. The Child Vaccination Histories File is provided with the public use data in order to make the calculation of exposure amounts transparent, and so that analysts have the potential to calculate alternative measures of exposure1. -

Gsk Vaccines in 2010
GSK VACCINES IN 2010 Thomas Breuer, MD, MSc Senior Vice President Head of Global Vaccines Development GSK Biologicals Vaccines business characteristics Few global players and high barriers to entry – Complex manufacturing – Large scale investment Long product life cycles – Complex intellectual property High probability of R&D success – 70% post-POC New technology/novel products Better pricing for newer vaccines – HPV vaccines (Cervarix, Gardasil) – Pneumococcal vaccines (Synflorix, Prevnar-13) Operating margin comparable to pharmaceutical products Heightened awareness New markets 2 Research & development timelines Identify Produce Pre-Clinical Proof of Registration/ Phase I Phase II Phase III File Antigens Antigens Testing Concept Post Marketing Research (inc. Immunology) Pre-Clinical Development (inc. Formulation Science) Clinical Development (inc. Post Marketing Surveillance) Transfer Process to Manufacturing Build Facility x x Up to $10-20M Up to $50-100M $500M - $1B x x x 1-10 yrs 2-3 yrs 2-4 yrs > 1 yr GSK vaccines business 2009 sales £3.7 billion (+30%) +19% CAGR excl. H1N1 Vaccines represent 13% since 2005 of total GSK sales Sale s (£m) 4000 3500 Recent approvals: 3000 US: Cervarix 2500 EU: Synflorix 2000 Pandemic: Pandemrix; Arepanrix 1500 1000 500 0 2005 2006 2007 2008 2009 Increased Emerging Market presence Growth rate is CER 5 GSK vaccines: fastest growing part of GSK in 2009 2009 Sales Share Growth (CER) Respiratory £ 6,977m 25% +5% Consumer £ 4,654m 16% +7% Anti-virals £ 4,150m 15% +12% Vaccines £ 3,706m 13% +30% CV & Urogenital -
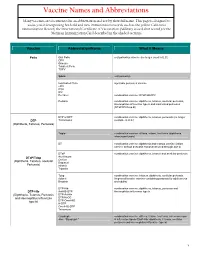
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many Vaccines Are Documented in an Abbreviation and Not by Their Full Name
Vaccine Names and Abbreviations Vaccine Names and Abbreviations Many vaccines are documented in an abbreviation and not by their full name. This page is designed to assist you in interpreting both old and new immunization records such as the yellow California Immunization Record, the International Certificate of Vaccination (Military issued shot record) or the Mexican Immunization Card described in the shaded sections. Vaccine Abbreviation/Name What it Means Polio Oral Polio oral poliovirus vaccine (no longer used in U.S.) OPV Orimune Trivalent Polio TOPV Sabin oral poliovirus Inactivated Polio injectable poliovirus vaccine eIPV IPOL IPV Pentacel combination vaccine: DTaP/Hib/IPV Pediarix combination vaccine: diphtheria, tetanus, acellular pertussis, Haemophilus influenzae type b and inactivated poliovirus (DTaP/IPV/Hep B) DTP or DPT combination vaccine: diphtheria, tetanus, pertussis (no longer DTP Tri-Immunol available in U.S.) (Diphtheria, Tetanus, Pertussis) Triple combination vaccine: difteria, tétano, tos ferina (diphtheria, tetanus pertussis) DT combination vaccine: diphtheria and tetanus vaccine (infant vaccine without pertussis component used through age 6) DTaP combination vaccine: diphtheria, tetanus and acellular pertussis DTaP/Tdap Acel-Imune Certiva (Diphtheria, Tetanus, acellular Daptacel Pertussis) Infanrix Tripedia Tdap combination vaccine: tetanus, diphtheria, acellular pertussis Adacel (improved booster vaccine containing pertussis for adolescents Boostrix and adults) DTP-Hib combination vaccine: diphtheria, tetanus, -

ACIP Meeting Agenda June 2018
Centers for Disease Control and Prevention 1600 Clifton Road, NE, Tom Harkin Global Communications Center, Kent "Oz" Nelson Auditorium Atlanta, Georgia 30329 June 20-21, 2018 AGENDA ITEM PURPOSE PRESIDER/PRESENTER(s) Wednesday, June 20 8:00 Welcome & Introductions Dr. Nancy Bennett (ACIP Chair) Dr. Amanda Cohn (ACIP Executive Secretary; CDC) 8:30 Influenza Vaccines Introduction Dr. Chip Walter (ACIP, WG Chair) VE update Dr. Brendan Flannery (CDC/NCIRD), Dr. Yun Lu (FDA) 2017-2018 influenza season vaccine safety update Dr. Tom Shimabukuro (CDC/NCEZID) Information Narcolepsy following adjuvanted monovalent pandemic H1N1 & influenza vaccines: Results of the SOMNIA study Dr. Tom Shimabukuro (CDC/NCEZID) Discussion Study results of an adjuvanted Quadravalent Influenza vaccine Dr. Gregg Sylvester (Seqirus) in young children 2018-19 recommendations Dr. Lisa Grohskopf (CDC/NCIRD) Public Comment Vote Vote Dr. Lisa Grohskopf (CDC/NCIRD) 10:40 Break 11:00 Anthrax Vaccines Introduction Dr. David Stephens (ACIP, WG Chair) GRADE Information Dr. William Bower (CDC/NCEZID) Summary of Work Group considerations and proposed policy & Dr. William Bower (CDC/NCEZID) options Discussion Public comment Vote Vote Dr. William Bower (CDC/NCEZID) 12:15 Lunch 1:30 HPV Vaccines Introduction Dr. Peter Szilagyi (ACIP, WG Chair) Information Current issues and background Dr. Lauri Markowitz (CDC/NCIRD) & HPV vaccine in mid-adults: results from clinical studies Dr. Alain Luxembourg (Merck) Discussion Considerations and Work Group plans ACIP HPV Vaccines Workgroup 2:40 Update on NITAGS Introduction Dr. Abigail Shefer (CDC/GID) Global NITAG activities and the GNN Information Dr. Joachim Hombach (Executive Secretary SAGE, WHO IVB) 3:10 Break 3:40 Mumps Vaccine Introduction Dr. -

Vaccine-Associated Paralytic Poliomyelitis and BCG-Osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013
Morbidity and Mortality Weekly Report Weekly / Vol. 63 / No. 33 August 22, 2014 Vaccine-Associated Paralytic Poliomyelitis and BCG-osis in an Immigrant Child with Severe Combined Immunodeficiency Syndrome — Texas, 2013 Robert Trimble, MD1, Jane Atkins, MD2, Troy C. Quigg, DO3, Cara C. Burns, PhD4, Gregory S. Wallace, MD4, Mary Thomas, MBBS5, Anil T. Mangla, PhD5, Anthony J. Infante, MD, PhD1 (Author affiliations at end of text) Poliovirus transmission has been eliminated in most of developed VAPP (2). A case of immunodeficiency-associated the world through the use of inactivated poliovirus vaccine vaccine-derived poliovirus (iVDPV) infection, without paraly- (IPV) and live, attenuated oral poliovirus vaccine (OPV). In sis, was diagnosed in an unvaccinated child with SCIDS in the United States, use of OPV was discontinued by the year 2005, but the source of the virus could not be definitively 2000 because of the potential for vaccine-associated paralytic identified (3). A woman in Minnesota aged 44 years with polio (VAPP); an average of eight cases were reported each long-standing common variable immunodeficiency died after year in the United States during 1980–2000 (1). Polio eradi- developing VAPP in 2009 (4). She was probably infected when cation efforts in other parts of the world continue to rely on her child received OPV approximately 12 years earlier. Case OPV to take advantage of transmission of poliovirus vaccine reports and cohort studies from several countries other than strains to unvaccinated persons in the population, lower cost, the United States demonstrate the continued occurrence of and ease of administration. In 2013, an infant aged 7 months iVDPVs and the need for ongoing surveillance (5).