Endemic Philippine Teak (Tectona Philippinensis Benth. & Hook
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Tectona Grandis Teak
Tectona grandis Teak Family: Verbenaceae Other Common Names: Kyun (Burma), Teck (French). Teca (Spanish). Distribution: Native to India, Burma, Thailand, Indochina, including Indonesia, particularly Java. Extensively cultivated in plantations within its natural range as well as in tropical areas of Africa and Latin America. The Tree On favorable sites, may reach 130 to 150 ft in height with clear boles to 80 to 90 ft; trunk diameters usually 3 to 5 ft; older trees fluted and buttressed. The Wood General Characteristics: Heartwood dark golden yellow, turning a dark brown with exposure, often very variable in color when freshly machined showing blotches and streaks of various shades; sapwood pale yellowish, sharply demarcated. Grain straight, sometimes wavy; texture coarse, uneven (ring porous); dull with an oily feel; scented when freshly cut. Dust may cause skin irritations. Silica content variable, up to 1.4% is reported. Weight: Basic specific gravity (ovendry weight/green volume) 0.55; air-dry density 40 pcf. Mechanical Properties: (First set of data based on the 2-cm standard; second and third sets on the 2-in. standard; third set plantation-grown in Honduras.) Moisture content Bending strength Modulus of elasticity Maximum crushing strength Psi 1,000 psi Psi Green (/7) 12,200 1,280 6.210 11% 15,400 1.450 8.760 Green (38) 10.770 1.570 5.470 14% 12,300 1.710 6.830 Green (81) 9.940 1.350 4.780 13% 13.310 1,390 6.770 Janka side hardness 1,000 to 1,155 lb for dry material. Forest Products Laboratory toughness 116 in.-lb average for green and dry wood (5/8-in. -

Bioresources.Com
PEER-REVIEWED ARTICLE bioresources.com EVALUATION AND IDENTIFICATION OF WALNUT HEARTWOOD EXTRACTIVES FOR PROTECTION OF POPLAR WOOD Seyyed Khalil Hosseini Hashemi a,* and Ahmad Jahan Latibari a Walnut (Juglans regia L.) heartwood extractives were identified and their potential for protection of poplar wood was evaluated. Test specimens were prepared from poplar wood (Populus nigra L.) to meet BS 838:1961 requirements. Samples were impregnated with heartwood extractive solution (1.5, 2.5, and 3.5% w/w in ethanol-toluene), followed by 5 hours vacuum desiccator technique to reach complete saturation. Impregnated specimens were exposed to white-rot fungus (Trametes versicolor) for 14 weeks according to BS 838:1961 applying the kolle- flask method. The weight loss of samples was determined after exposure to white-rot fungus. The highest weight loss (36.96%) was observed for untreated control samples and the lowest weight loss (30.40%) was measured in samples treated with 1.5% extractives solution. The analyses of the extracts using GC/MS indicated that major constituents are benzoic acid,3,4,5-tri(hydroxyl) and gallic acid (44.57 %). The two toxic components in the heartwood are juglone (5.15 %) and 2,7- dimethylphenantheren (5.81 %). Keywords: Walnut heart wood extractives; Trametes versicolor; Weight loss; Gallic acid; Juglone; 2,7- dimethylphenantheren Contact information; a: Agriculture Research Center, Islamic Azad University, Karaj Branch, P. O. Box 331485-313, Karaj, Iran. * Corresponding author: [email protected] INTRODUCTION One of the promising strategies to slow down the decay and deterioration of wood structure is to rely upon durable wood species. -
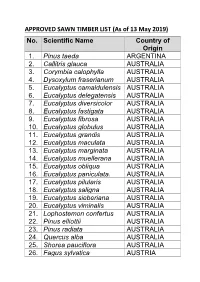
APPROVED SAWN TIMBER LIST (As of 13 May 2019) No. Scientific Name Country of Origin 1
APPROVED SAWN TIMBER LIST (As of 13 May 2019) No. Scientific Name Country of Origin 1. Pinus taeda ARGENTINA 2. Callitris glauca AUSTRALIA 3. Corymbia calophylla AUSTRALIA 4. Dysoxylum fraserianum AUSTRALIA 5. Eucalyptus camaldulensis AUSTRALIA 6. Eucalyptus delegatensis AUSTRALIA 7. Eucalyptus diversicolor AUSTRALIA 8. Eucalyptus fastigata AUSTRALIA 9. Eucalyptus fibrosa AUSTRALIA 10. Eucalyptus globulus AUSTRALIA 11. Eucalyptus grandis AUSTRALIA 12. Eucalyptus maculata AUSTRALIA 13. Eucalyptus marginata AUSTRALIA 14. Eucalyptus muellerana AUSTRALIA 15. Eucalyptus obliqua AUSTRALIA 16. Eucalyptus paniculata. AUSTRALIA 17. Eucalyptus pilularis AUSTRALIA 18. Eucalyptus saligna AUSTRALIA 19. Eucalyptus sieberiana AUSTRALIA 20. Eucalyptus viminalis AUSTRALIA 21. Lophostemon confertus AUSTRALIA 22. Pinus elliottii AUSTRALIA 23. Pinus radiata AUSTRALIA 24. Quercus alba AUSTRALIA 25. Shorea pauciflora AUSTRALIA 26. Fagus sylvatica AUSTRIA No. Scientific Name Country of Origin 27. Picea abies AUSTRIA 28. Picea abies BELARUS 29. Pinus sylvestris BELARUS 30. Quercus alba BELGIUM 31. Dipteryx odorata BOLIVIA 32. Apuleia leiocarpa BRAZIL 33. Astronium lecointei BRAZIL 34. Bagassa guianensis BRAZIL 35. Cedrela odorata BRAZIL 36. Cedrelinga catenaeformis BRAZIL 37. Couratari guianensis BRAZIL 38. Dipteryx odorata BRAZIL 39. Eucalyptus grandis BRAZIL 40. Eucalyptus grandis BRAZIL 41. Hymenaea courbaril BRAZIL 42. Hymenolobium modestum BRAZIL 43. Hymenolobium Nitidum BRAZIL Benth 44. Hymenolobium BRAZIL pulcherrimum 45. Manilkara bidentata BRAZIL 46. Myroxylon balsamum BRAZIL 47. Pinus radiata BRAZIL 48. Pinus taeda BRAZIL 49. Quassia simarouba BRAZIL 50. Tectona grandis BRAZIL 51. Fagus sylvatica BULGARIA No. Scientific Name Country of Origin 52. Quercus alba BULGARIA 53. Chlorophora excelsa CAMEROON 54. Cylicodiscus gabunensis CAMEROON 55. Entandrophragma CAMEROON candollei 56. Entandrophragma CAMEROON cylindricum 57. Entandrophragma CAMEROON cylindricum 58. Entandrophragma utile CAMEROON 59. -
Dry Kiln Operator's Manual
United States Department of Agriculture Dry Kiln Forest Service Operator's Forest Products Laboratory Manual Madison, Wisconsin Agriculture Handbook No. 188 Dry Kiln Operator’s Manual Edited by William T. Simpson, Research Forest Products Technologist United States Department of Agriculture Forest Service Forest Products Laboratory 1 Madison, Wisconsin Revised August 1991 Agriculture Handbook 188 1The Forest Products Laboratory is maintained in cooperation with the University of Wisconsin. This publication reports research involving pesticides. It does not contain recommendations for their use, nor does it imply that the uses discussed here have been registered. All uses of pesticides must be registered by appropriate State and/or Federal agencies before they can be recommended. CAUTION, Pesticides can be injurious to humans, domestic animals, desirable plants, and fish or other wildlife-if they are not handled or applied properly. Use all pesticides selectively and carefully. Follow recommended practices for the disposal of surplus pesticides aand pesticide containers. Preface Acknowledgments The purpose of this manual is to describe both the ba- Many people helped in the revision. We visited many sic and practical aspects of kiln drying lumber. The mills to make sure we understood current and develop- manual is intended for several types of audiences. ing kiln-drying technology as practiced in industry, and First and foremost, it is a practical guide for the kiln we thank all the people who allowed us to visit. Pro- operator-a reference manual to turn to when questions fessor John L. Hill of the University of New Hampshire arise. It is also intended for mill managers, so that they provided the background for the section of chapter 6 can see the importance and complexity of lumber dry- on the statistical basis for kiln samples. -

A Guide to Lesser Known Tropical Timber Species July 2013 Annual Repo Rt 2012 1 Wwf/Gftn Guide to Lesser Known Tropical Timber Species
A GUIDE TO LESSER KNOWN TROPICAL TIMBER SPECIES JULY 2013 ANNUAL REPO RT 2012 1 WWF/GFTN GUIDE TO LESSER KNOWN TROPICAL TIMBER SPECIES BACKGROUND: BACKGROUND: The heavy exploitation of a few commercially valuable timber species such as Harvesting and sourcing a wider portfolio of species, including LKTS would help Mahogany (Swietenia spp.), Afrormosia (Pericopsis elata), Ramin (Gonostylus relieve pressure on the traditionally harvested and heavily exploited species. spp.), Meranti (Shorea spp.) and Rosewood (Dalbergia spp.), due in major part The use of LKTS, in combination with both FSC certification, and access to high to the insatiable demand from consumer markets, has meant that many species value export markets, could help make sustainable forest management a more are now threatened with extinction. This has led to many of the tropical forests viable alternative in many of WWF’s priority places. being plundered for these highly prized species. Even in forests where there are good levels of forest management, there is a risk of a shift in species composition Markets are hard to change, as buyers from consumer countries often aren’t in natural forest stands. This over-exploitation can also dissuade many forest willing to switch from purchasing the traditional species which they know do managers from obtaining Forest Stewardship Council (FSC) certification for the job for the products that they are used in, and for which there is already their concessions, as many of these high value species are rarely available in a healthy market. To enable the market for LKTS, there is an urgent need to sufficient quantity to cover all of the associated costs of certification. -

Chapter 3--Physical Properties and Moisture Relations of Wood
Chapter 3 Physical Properties and Moisture Relations of Wood William Simpson and Anton TenWolde he versatility of wood is demonstrated by a wide Contents variety of products. This variety is a result of a Appearance 3–1 spectrum of desirable physical characteristics or properties among the many species of wood. In many cases, Grain and Texture 3–1 more than one property of wood is important to the end Plainsawn and Quartersawn 3–2 product. For example, to select a wood species for a product, the value of appearance-type properties, such as texture, grain Decorative Features 3–2 pattern, or color, may be evaluated against the influence of Moisture Content 3–5 characteristics such as machinability, dimensional stability, Green Wood and Fiber Saturation Point 3–5 or decay resistance. Equilibrium Moisture Content 3–5 Wood exchanges moisture with air; the amount and direction of the exchange (gain or loss) depend on the relative humid- Sorption Hysteresis 3–7 ity and temperature of the air and the current amount of water Shrinkage 3–7 in the wood. This moisture relationship has an important Transverse and Volumetric 3–7 influence on wood properties and performance. This chapter discusses the physical properties of most interest in the Longitudinal 3–8 design of wood products. Moisture–Shrinkage Relationship 3–8 Some physical properties discussed and tabulated are influ- Weight, Density, and Specific Gravity 3–11 enced by species as well as variables like moisture content; Working Qualities 3–15 other properties tend to be independent of species. The thor- oughness of sampling and the degree of variability influence Decay Resistance 3–15 the confidence with which species-dependent properties are Thermal Properties 3–15 known. -

Characterisation and Fractioning of Tectona Grandis Bark in View of Its
Industrial Crops and Products 50 (2013) 166–175 Contents lists available at ScienceDirect Industrial Crops and Products journa l homepage: www.elsevier.com/locate/indcrop Characterisation and fractioning of Tectona grandis bark in view of its valorisation as a biorefinery raw-material a a a,b a,∗ a Isabel Baptista , Isabel Miranda , Teresa Quilhó , Jorge Gominho , Helena Pereira a Centro de Estudos Florestais, Instituto Superior de Agronomia, Universidade Técnica de Lisboa, Tapada da Ajuda, 1349-017 Lisboa, Portugal b Centro das Florestas e Produtos Florestais, Instituto de Investigac¸ ão Científica Tropical, Tapada da Ajuda, 1349-017 Lisboa, Portugal a r t a b i s c l e i n f o t r a c t Article history: The anatomy and chemical composition of Tectona grandis bark from mature trees in East Timor Received 6 March 2013 are described as well as the characterisation of fractionation by grinding and granulometric Received in revised form 5 June 2013 separation. Accepted 2 July 2013 Teak bark is composed of secondary phloem, periderm and a narrow rhytidome that included various periderms with phloem tissues between them. The layer of phellem cells in each periderm was thin. Keywords: The phloem showed an orderly stratification with tangential bands of fibres in concentric rings that Tectona grandis alternated with thin bands of axial parenchyma and sieve tube elements. Abundant prismatic calcium Teak bark Anatomy oxalate crystals were present. The bark fractured easily into clean particles. The yield of fines was low and 64.4% of the particles were Chemical composition Fractionation over 2 mm. The mean chemical composition of teak bark was: ash 18.5%, total extractives 10.7%, lignin 20.0% and suberin 1.9%. -

Hardwood.Pdf
Sorted by name Sorted by hardness Species Hardness Species Hardness Species Hardness Species Hardness Species Hardness Species Hardness African Ebony 3220 Carribean Rosewood 3170 Patagonian Rosewood 2800 Brazilian Walnut 3684 Pyinkado 1950 Maple 1450 African Hickory 1820 Chinese Hickory 1820 Pearwood 2990 Ebonya Walnut 3684 African Merbau 1925 Brazilian Mahogany 1400 African Kempas 1710 Crassiflora 3220 Pecan 1820 Ipe 3684 Bijuga 1925 Mahogany 1400 African Merbau 1925 Dark Rosewood 2300 Pine 870 African Ebony 3220 Joemoek 1925 Spanish Mahogany 1400 African Paduak 1725 Ebony 3220 Pyinkado 1950 Crassiflora 3220 Kayu Kuku 1925 Oak 1360 African Rosewood 1780 Ebonya Walnut 3684 Red Oak 1290 Ebony 3220 Merbau 1925 White Oak 1360 African Sapele 1510 Elm 800 Rojo Mahogany 2200 Tanzinia Msindi 3220 West Indies Walnut 1925 Ash 1320 Afzelia 1810 Erable 1450 Rosewood 1650 Brazilian Rosewood 3200 Amendoim 1912 Beech 1300 Amendoim 1912 European Teak 1155 Santos Mahogany 2200 Tiete Rosewood 3200 Brazilian Oak 1912 Red Oak 1290 American Cherry 950 Havea 1820 Sao Paulo Striped Wood 2160 Carribean Rosewood 3170 African Hickory 1820 Birch 1260 Applewood 880 Heart Pine 870 Sapele 1510 Huali 3170 Carribean Pecan 1820 Iroko 1260 Argentinian Chestnut 2670 Hevea 1820 Simianping 3170 Simianping 3170 Chinese Hickory 1820 Merisier 1260 Ash 1320 Hickory 1820 Spanish Mahogany 1400 Lapacho 3060 Havea 1820 Yellow Birch 1260 Asian Teak 1155 Hickory/Pecan 1820 Sucupira 2140 Taheebo 3060 Hevea 1820 Bamboo 1180 Asian Walnut 1010 Huali 3170 Tadenia 1570 Pearwood 2990 Hickory -

Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 5, Issue 3, 2013
Academic Sciences International Journal of Pharmacy and Pharmaceutical Sciences ISSN- 0975-1491 Vol 5, Issue 3, 2013 Research Article PHYTOCHEMICAL AND PHARMACOLOGICAL EVALUATION OF TECTONA GRANDIS.LINN NEHA KHERAa* AND SANGEETA BHARGAVAb a*Department of Chemistry, University of Rajasthan, Jaipur, India, bDepartment of Chemistry, University of Rajasthan, Jaipur, India. Email: [email protected] Received: 20 May 2013, Revised and Accepted: 22 Mar 2013 ABSTRACT Tectona grandis Linn. commonly known as Teak or sagwan is one of the most famous timber in the world and is renowned for its dimensional stability. Teak is a major exotic species found in tropical region. It is commonly found in India and other south Asian countries. Teak is also considered as a major constituent in many folklore medicines. Medicinally it has various pharmacological activities like antibacterial, antioxidant, antifungal, anti-inflammatory, anti-pyretic, analgesic, anti-diuretic, and hypoglycemic. Further studies reveal the presence of carbohydrate, tannins, alkaloids, saponins, proteins and flavonoids. Extracts from various parts of teak is used to cure bronchitis, biliousness, dysentery, diabetes, leprosy, hyperacidity. Its medicinal properties are recognized in ayurvedic system of medicine. Keyword: Tectona grandis, Antioxidant, Antifungal, Phenolic compounds, Diabetes. INTRODUCTION Marwadi: Sagwan, Sag. Tectona grandisLinn. (TG) is commonly known as “teak” belongs to Punjabi: Sagwan, Sagun. verbanaceae family. It is a large deciduous tree 30-35 metre tall with light brown bark,leaves simple, opposite, broadly elliptical or acute Tamil: Tekku, Tekkumaram, Tek, Kalindi. or acuminate, with minute glandular dots, flowers are white in Telgu: Teku, Pedda, Tek, Peddateku, teku-manu, Adaviteku, Teechekka. colour, small and have a pleasant smell[1]. -

Swietenia Macrophylla Meliaceae King
Swietenia macrophylla King Meliaceae mahogany LOCAL NAMES Bengali (bara mahauni,bara-mahagoni,mahagni); Dutch (mahonie,mahok); English (Dominican mahogany,bastard mahogany,big- leaf mahogany,Brazilian mahogany tree,Colombian mahogany tree,Honduras mahogany,large-leaved mahogany,Mexican mahogany tree,West Indian mahogany,Spanish mahogany,mahogany,Peruvian mahogany tree); French (acajou du Venezuela,acajou étranger,acajou du Honduras); German (Echtes mahagoni); Italian (mogano); Malay (cheria mahogany); Portuguese (mogno); Spanish (caoba de Santo,domingo,Caoba de Honduras,caoba del Atlántico,caoba Forest giant escaping extraction from hondureña,zopilozontecomacuahuitl,caoba); Trade name (mahogany) loggers near Maraba, Brazil. (Anthony Simons) BOTANIC DESCRIPTION Swietenia macrophylla is a very large tree, reaching a height of 30-40 m and a girth of 3-4 m; in favourable conditions it can reach 60 m high and 9 m girth. Trunk straight, cylindrical, with a buttressed base; bark rough, flaking off in small patches. Leaves paripinnate, up to 60 cm long; leaflets 6-16, ovate, lanceolate, acuminate, slightly oblique, light green or reddish when young, dark green and shining when mature, up to 20 cm long, with 8-12 pale, secondary nerves. Trees grown in mixed agroforest plot in Davao, Philippines (Anthony Simons) Flowers 8 mm across, in narrow supra-axillary panicles about 8-13 cm long and fragrant; petals greenish-white, oblong, 4 mm long, rigidly pointed. Fruit a woody capsule resembling a large inverted club, about 12.5 x 7.5 cm, erect. ‘Swietenia’ commemorates Gerard von Swieten (1700-1772), botanist and physician to Maria Theresa of Austria. The specific name, ‘macrophylla’, means large leaved and comes from Greek words ‘makros’ (large) and ‘phyllon’ (leaf). -

FALSE RING OCCURRENCES and THEIR IDENTIFICATION in TEAK (Tectona Grandis) in NORTH-EASTERN THAILAND
Journal of Tropical Forest Science 24(3): 387–398 (2012) Palakit K et al. FALSE RING OCCURRENCES AND THEIR IDENTIFICATION IN TEAK (TECTONA GRANDIS) IN NORTH-EASTERN THAILAND K Palakit1, 2, S Siripattanadilok3 & K Duangsathaporn2, 4 1Graduate School of Kasetsart University, Bangkok, Thailand, 10900. E-mail: [email protected] 2Center for Advanced Studies in Tropical Natural Resources, National Research University–Kasetsart University, Kasetsart University, Chatuchak, Bangkok, Thailand, 10900 3Department of Forest Biology, Faculty of Forestry, Kasetsart University, Bangkok, Thailand, 10900 4Department of Forest Management, Faculty of Forestry, Kasetsart University, Bangkok, Thailand, 10900 Received August 2011 PALAKIT K, SIRIPATTANADILOK S & DUANGSATHAPORN K. 2012. False ring occurrences and their identification in teak (Tectona grandis) in north-eastern Thailand. The objectives of this research were to identify and locate the position of false ring occurrences in natural teak (Tectona grandis) and to relate their formation to local climate variability. Vessel diameters were measured and standardised from pith towards the bark in order to identify false rings and define annual ring boundaries. Two types of false rings were classified as false ring type I and II in earlywood and latewood respectively. False ring type I had one or more rows of axial parenchyma associated with large vessels at the beginning of the annual ring. False ring type II was divided into two groups based on their characteristics. The first group had an aggregation of large vessels associated with paratracheal parenchyma while the second group did not have any paratracheal parenchyma. The occurrences of false rings could be explained by the fluctuations of rainfall and temperature during the growing season. -

Threats on the Natural Stand of Philippine Teak Along Verde Island Passage Marine Corridor JESAM (VIPMC), Southern Luzon, Philippines
54 Journal of Environmental Science and Management 20-2: 54-67 (December 2017) ISSN 0119-1144 Threats on the Natural Stand of Philippine Teak along Verde Island Passage Marine Corridor JESAM (VIPMC), Southern Luzon, Philippines ABSTRACT This study documents the threats of the critically endangered Tectona Romel U. Briones1* philippinensis in the backdrop of the past conservation policies and projects. Twelve Edwin R. Tadiosa2 20m x 50m plots were distributed in three altitudinal strata (S1= 50 – 100 m asl, S2= Antonio C. Manila3 150 to 200 m asl, and S3= 250 – 300 m asl) using stratified random sampling. Every tree was examined to detect presence of pest and diseases on foliage, stem, buttress 1 Tropical Forestry Department and exposed root system. Threats of anomalous weather patterns like intense drought College of Agriculture and Forestry – and human disturbances were also recorded. Leaf skeletonizers, shotholes, buttrot, BatStateU Lobo Campus heartrot, rootrot, illegal harvesting, charcoal making, wind damages, and intense dry Batangas State University, season are among the most alarming threats of T. philippinensis. Germinants and Rizal Avenue, Batangas City, wildlings are most susceptible to wilting during intense drought during dry season. A Philippines 4200 number of interesting species of arthropods and macrofungi within the stand were also 2 Philippine National Herbarium encountered. There are variations on the incidence and infection across altitudinal Botany Division, National Museum of habitat and across diameter classes. Poles and standards at lower altitudinal habitat the Philippines, Manila (<100 m asl) are the most disturbed and susceptible to the disturbances. Existing 3 Biodiversity Management Bureau conservation and protection policies should be strictly implemented especially in Ninoy Aquino Parks and Wildlife hotspot habitats.