Petg Chemical Resistance
Total Page:16
File Type:pdf, Size:1020Kb
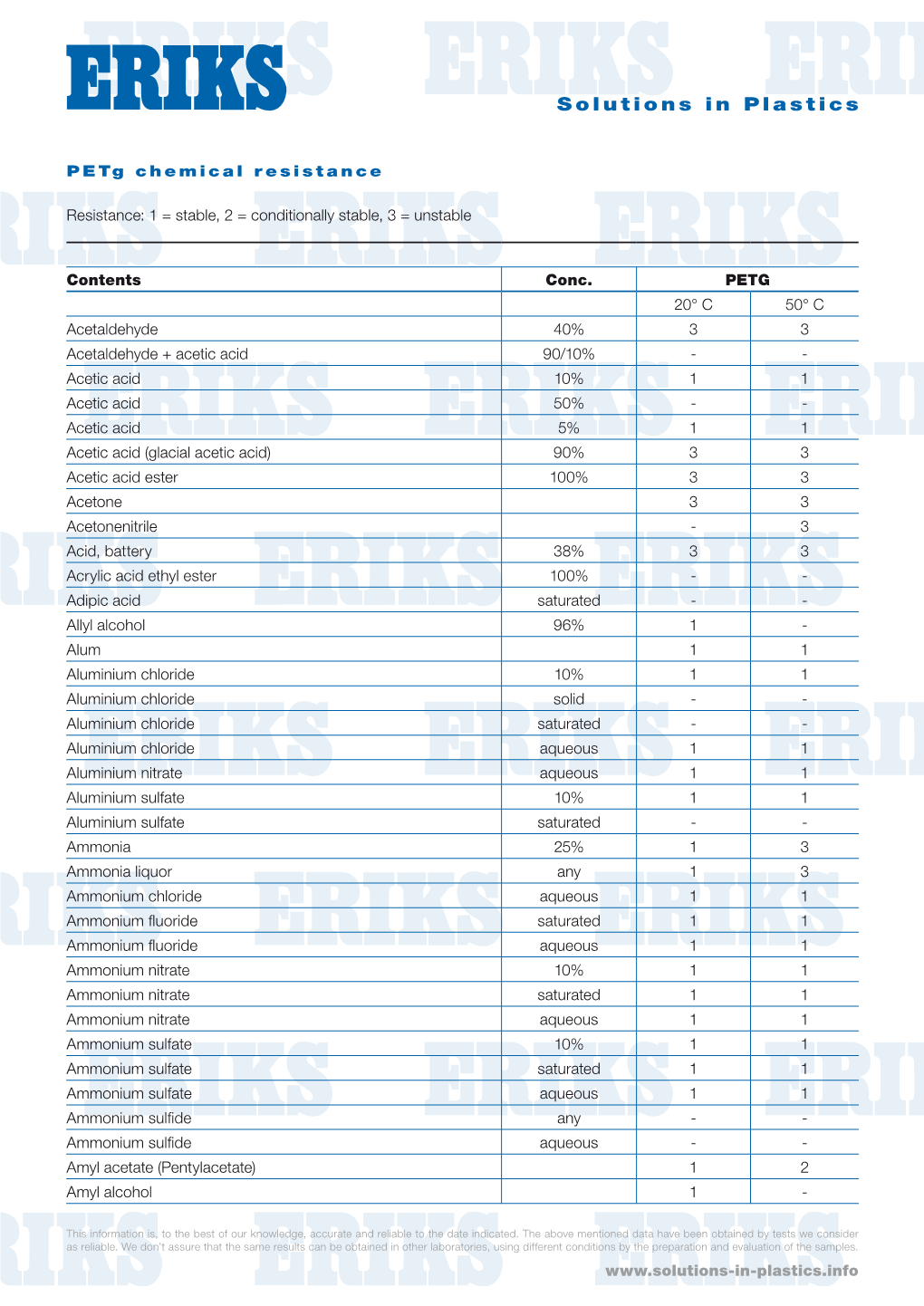
Load more
Recommended publications
-

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

Methanol Synthesis Catalyst
Office europeen des brevets (fi) Publication number : 0 482 753 A2 @ EUROPEAN PATENT APPLICATION @ Application number: 91308468.7 @ Int. CI.5: B01J 23/80, B01J 23/84, B01J 23/88, C07C 29/154 @^ Date of filing : 17.09.91 Claim 16 is deemed to be abandoned due to (72) Inventor : Sofianos, Alkeos non-payment of the claim fee (Rule 31 (2) EPC). 253 Asimptote Street, Meyerspark Pretoria, Transvaal Province (ZA) (§) Priority : 18.09.90 ZA 907437 (74) Representative : Spall, Christopher John @ Date of publication of application : BARKER, BRETTELL & DUNCAN 138 Hagley 29.04.92 Bulletin 92/18 Road Edgbaston Birmingham B16 9PW (GB) @ Designated Contracting States : AT BE CH DE DK ES FR GB GR IT LI LU NL SE (7i) Applicant : CSIR Corporate Building, Scientia Pretoria Transvaal Province (ZA) (54) Methanol synthesis catalyst. (57) A method of forming a methanol synthesis catalyst precursor comprises forming a precipi- tate comprising compounds, thermally decom- posable to oxides or mixed oxides, of copper, zinc, aluminium and at least one element of Group IVB and/or Group VIIB of the Periodic Table of Elements. CM < CO If) h- CM 00 LU Jouve, 18, rue Saint-Denis, 75001 PARIS EP 0 482 753 A2 THIS INVENTION relates to the synthesis of methanol. It relates in particular to a methanol synthesis catal- yst precursor and to a method of forming such precursor; to a methanol synthesis catalyst and to a method of making such a catalyst; to an active methanol synthesis catalyst, and to a method of making thereof; and to a methanol synthesis process. -

Combustion Synthesis of Nanocrystalline Al2o3 Powder
Available online a t www.pelagiaresearchlibrary.com Pelagia Research Library Advances in Applied Science Research, 2012, 3 (6):3819-3824 ISSN: 0976-8610 CODEN (USA): AASRFC Combustion Synthesis of Nanocrystalline Al 2O3 Powder using Aluminium Nitrate and Urea as reactants—influence of reactant composition Amit Sharma *, O. P. Modi ** and Gourav K Gupta** *Department of Mechanical Engineering, Arni School of Technology, Arni University, Kathgarh, Indora, (H.P.)-176401. ** Advanced Materials and Processes Research Institute (CSIR), Bhopal _____________________________________________________________________________________________ ABSTRACT Combustion synthesis technique for synthesis of Aluminium oxide using urea as fuel and Aluminium Nitrate as an oxidizer is found to be easy & economical route & is able to produce Nano phase alumina powder. In present experiment urea is found to have outstanding potential towards solution combustion. Exothermicity is excellent and exothermic flame temperature increase with increase in oxidizer/ fuel ratio. A well defined crystalline structure and Nano scale particles size found after XRD and SEM analysis. Overall Urea-Nitrate combustion synthesis has an outstanding potential for producing pure Alumina powder. Crystallinity is excellent in case of urea, which leads to use in applications require high strength crystalline material. Keywords: Metal oxide, ceramic oxides, Combustion synthesis, SHS (Self propagating high temperature synthesis), Solution Combustion, Nano-ceramics. _____________________________________________________________________________________________ -

Radiochemical Assessment of Uranium in Locally Used Marbles
Journal of the Institute of Engineering 200 TUTA/IOE/PCU January 2019, Vol. 15 (No. 1):cl 200-209 © TUTA/IOE/PCU, Printed in Nepal Radiochemical Assessment of Uranium in Locally Used Marbles Birendra Thapa1, Kamal Krishna Shrestha2a, Khagendra Prasad Bohara2 1Department of Applied Sciences, Pulchowk Campus, Institute of Engineering, TU, Nepal 2Central Department of Chemistry, Tribhuvan University, Kathmandu, Nepal Corresponding author: [email protected] Received: Nov 5, 2018 Revised: Jan 1, 2019 Accepted: Jan 3, 2019 Abstract: A sensitive method is used for the extraction and determination of soluble uranium in locally used marble has been developed. Isobutyl Methyl Ketone (IBMK) was used as extractant and 0.02 % (w/v) Arsenazo (III) as a chromogenic reagent. The present study assesses the gross radioactivity and extractable uranium in marble samples. A Multichannel Analyzer (MCA) coupled with NaI (Tl) crystal detector was used for the measurement of gross radioactivity whereas spectrophotometer was used to estimate the uranium contained in sample with the help of Arsenazo (III). The extraction involved the adjustment of pH at 0.9. The efficient extraction was achieved by the addition of at (+2) least 10 g of Al (NO3)3. This method involved the reduction of UO2 to U (IV) by using granulated Zinc in concentrated hydrochloric acid. The physical measurements showed the presence of gross radioactivity in the range 266.19 Bq/kg to 644.268 Bq/Kg while the chemical analysis showed the uranium concentration in the range of 0.02ppm to 0.06 ppm. Key Words: Arsenazo (III), IBMK, multichannel analyzer, spectrophotometer, uranium 1. -

Opinion of the Scientific Committee on Consumer Safety on O
SCCS/1613/19 Final Opinion Version S Scientific Committee on Consumer Safety SCCS OPINION ON the safety of aluminium in cosmetic products Submission II The SCCS adopted this document at its plenary meeting on 03-04 March 2020 SCCS/1613/19 Final Opinion Opinion on the safety of aluminium in cosmetic products – submission II ___________________________________________________________________________________________ ACKNOWLEDGMENTS Members of the Working Group are acknowledged for their valuable contribution to this Opinion. The members of the Working Group are: For the preliminary and the final versions SCCS members Dr U. Bernauer Dr L. Bodin (Rapporteur) Prof. Q. Chaudhry (SCCS Chair) Prof. P.J. Coenraads (SCCS Vice-Chair and Chairperson of the WG) Prof. M. Dusinska Dr J. Ezendam Dr E. Gaffet Prof. C. L. Galli Dr B. Granum Prof. E. Panteri Prof. V. Rogiers (SCCS Vice-Chair) Dr Ch. Rousselle Dr M. Stepnik Prof. T. Vanhaecke Dr S. Wijnhoven SCCS external experts Dr A. Koutsodimou Dr A. Simonnard Prof. W. Uter All Declarations of Working Group members are available on the following webpage: http://ec.europa.eu/health/scientific_committees/experts/declarations/sccs_en.htm This Opinion has been subject to a commenting period of a minimum eight weeks after its initial publication (from 16 December 2019 until 17 February 2020). Comments received during this time period are considered by the SCCS. For this Opinion, some changes occurred, in particular in sections 1, 3.2, 3.3.4.5, 3.3.8.1, 3.5, as well as in related discussion parts and conclusion (question 1). The list of references has also been updated. -

Safety Data Sheet
SAFETY DATA SHEET Creation Date 05-Oct-2010 Revision Date 02-Feb-2021 Revision Number 3 SECTION 1: IDENTIFICATION OF THE SUBSTANCE/MIXTURE AND OF THE COMPANY/UNDERTAKING 1.1. Product identifier Product Description: Aluminum nitrate nonahydrate Cat No. : 43145 Synonyms Nitric acid, aluminum salt, nonahydrate.; Aluminum trinitrate nonahydrate CAS-No 7784-27-2 Molecular Formula Al N3 O9 . 9 H2 O Reach Registration Number - 1.2. Relevant identified uses of the substance or mixture and uses advised against Recommended Use Laboratory chemicals. Uses advised against No Information available 1.3. Details of the supplier of the safety data sheet Company Alfa Aesar . Avocado Research Chemicals, Ltd. Shore Road Port of Heysham Industrial Park Heysham, Lancashire LA3 2XY United Kingdom Office Tel: +44 (0) 1524 850506 Office Fax: +44 (0) 1524 850608 E-mail address [email protected] www.alfa.com Product Safety Department 1.4. Emergency telephone number Call Carechem 24 at +44 (0) 1865 407333 (English only); +44 (0) 1235 239670 (Multi-language) SECTION 2: HAZARDS IDENTIFICATION 2.1. Classification of the substance or mixture CLP Classification - Regulation (EC) No 1272/2008 Physical hazards Based on available data, the classification criteria are not met Health hazards ______________________________________________________________________________________________ ALFAA43145 Page 1 / 10 SAFETY DATA SHEET Aluminum nitrate nonahydrate Revision Date 02-Feb-2021 ______________________________________________________________________________________________ Serious Eye Damage/Eye Irritation Category 1 (H318) Environmental hazards Based on available data, the classification criteria are not met Full text of Hazard Statements: see section 16 2.2. Label elements Signal Word Danger Hazard Statements H318 - Causes serious eye damage Precautionary Statements P280 - Wear eye protection/ face protection P305 + P351 + P338 - IF IN EYES: Rinse cautiously with water for several minutes. -

Molybdenum Behaviour During U-Al Research Reactor Spent Fuel Dissolution X
molybdenum behaviour during u-al research reactor spent fuel dissolution X. Heres, D. Sans, E. Brackx, R. Domenger, M. Bertrand, E. Excoffier, C. Eysseric, Jf. Valery To cite this version: X. Heres, D. Sans, E. Brackx, R. Domenger, M. Bertrand, et al.. molybdenum behaviour during u-al research reactor spent fuel dissolution. RRFM 2017– European Research Reactor Conference, May 2017, Rotterdam, Netherlands. hal-02433875 HAL Id: hal-02433875 https://hal.archives-ouvertes.fr/hal-02433875 Submitted on 9 Jan 2020 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. RRFM 2017– European Research Reactor Conference Rotterdam, 14-18 May 2017 MOLYBDENUM BEHAVIOUR DURING U-AL RESEARCH REACTOR SPENT FUEL DISSOLUTION X. HÉRÈS *, D. SANS, E. BRACKX, R. DOMENGER, M. BERTRAND, E. EXCOFFIER, C. EYSSERIC, CEA, Nuclear Energy Division, Research Department on Mining and Fuel Recycling Processes BP 17171, F-30207 Bagnols sur Cèze, France J. F. VALERY AREVA NC, BUR/DT/RDP, La Défense, France ABSTRACT In the frame of Research Reactors Spent Fuel (RRSF) treatment by hydrometallurgy, the dissolution in nitric acid of irradiated U-Al, is a key issue because of the low solubility of molybdenum fission product in presence of high concentration of aluminium. -

Chemical Names and CAS Numbers Final
Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number C3H8O 1‐propanol C4H7BrO2 2‐bromobutyric acid 80‐58‐0 GeH3COOH 2‐germaacetic acid C4H10 2‐methylpropane 75‐28‐5 C3H8O 2‐propanol 67‐63‐0 C6H10O3 4‐acetylbutyric acid 448671 C4H7BrO2 4‐bromobutyric acid 2623‐87‐2 CH3CHO acetaldehyde CH3CONH2 acetamide C8H9NO2 acetaminophen 103‐90‐2 − C2H3O2 acetate ion − CH3COO acetate ion C2H4O2 acetic acid 64‐19‐7 CH3COOH acetic acid (CH3)2CO acetone CH3COCl acetyl chloride C2H2 acetylene 74‐86‐2 HCCH acetylene C9H8O4 acetylsalicylic acid 50‐78‐2 H2C(CH)CN acrylonitrile C3H7NO2 Ala C3H7NO2 alanine 56‐41‐7 NaAlSi3O3 albite AlSb aluminium antimonide 25152‐52‐7 AlAs aluminium arsenide 22831‐42‐1 AlBO2 aluminium borate 61279‐70‐7 AlBO aluminium boron oxide 12041‐48‐4 AlBr3 aluminium bromide 7727‐15‐3 AlBr3•6H2O aluminium bromide hexahydrate 2149397 AlCl4Cs aluminium caesium tetrachloride 17992‐03‐9 AlCl3 aluminium chloride (anhydrous) 7446‐70‐0 AlCl3•6H2O aluminium chloride hexahydrate 7784‐13‐6 AlClO aluminium chloride oxide 13596‐11‐7 AlB2 aluminium diboride 12041‐50‐8 AlF2 aluminium difluoride 13569‐23‐8 AlF2O aluminium difluoride oxide 38344‐66‐0 AlB12 aluminium dodecaboride 12041‐54‐2 Al2F6 aluminium fluoride 17949‐86‐9 AlF3 aluminium fluoride 7784‐18‐1 Al(CHO2)3 aluminium formate 7360‐53‐4 1 of 75 Chemical Abstract Chemical Formula Chemical Name Service (CAS) Number Al(OH)3 aluminium hydroxide 21645‐51‐2 Al2I6 aluminium iodide 18898‐35‐6 AlI3 aluminium iodide 7784‐23‐8 AlBr aluminium monobromide 22359‐97‐3 AlCl aluminium monochloride -

Table of Contents
Canadian Environmental Protection Act, 1999 PRIORITY SUBSTANCES LIST ASSESSMENT REPORT FOLLOW-UP TO THE STATE OF SCIENCE REPORT, 2000 Aluminum Chloride Aluminum Nitrate Aluminum Sulphate Chemical Abstracts Service Registry Numbers 7446-70-0 13473-90-0 10043-01-3 Environment Canada Health Canada January 2010 TABLE OF CONTENTS LIST OF FIGURES.............................................................................................................v LIST OF TABLES .............................................................................................................vi LIST OF ACRONYMS AND ABBREVIATIONS..........................................................vii SYNOPSIS.........................................................................................................................xi 1 INTRODUCTION........................................................................................................1 2 SUMMARY OF INFORMATION CRITICAL TO ASSESSMENT OF “TOXIC” UNDER CEPA 1999....................................................................................................5 2.1 Identity and physical/chemical properties.............................................................5 2.2 Entry characterization...........................................................................................6 2.2.1 Production, import, export and use ................................................................6 2.2.1.1 Aluminum chloride.................................................................................8 2.2.1.2 Aluminum -

Government of India Atomic Energy Commission
B.A.R.C.-638 "Ba^spjU' GOVERNMENT OF INDIA ATOMIC ENERGY COMMISSION SPECTROPHOTOMETRIC DETERMINATION OF URANIUM IN COMMERCIAL WET PROCESS PHOSPHORIC ACID AND PHOSPHATE ROCK USING THIOCYANATE by R. A. Nagle and T. K. S. Murthy Chemical Engineering Division BHABHA ATOMIC RESEARCH CENTRE BOMBAY, INDIA 1972 We regret that some of the pages in the microfiche copy of this report may not be up to the proper legibility standards, evea though the best possible copy was used for preparing the master fiche. B.A .B.C.-630 GOVERNMENT OF INDIA co ATOMIC ENERGY COMMISSION to o « « < SPECTROBiOKMETRIC DETERMINATION OF URANIUM IN COMMERCIAL WET PROCESS PHOSPHORIC? ACID AND PHOSPHATE RUCK USING TRIOCYANATE by R.A. Nagle and V.K.S. Murthy Chemical Engineerirg Division 1-JHABHA ATOMIC RESEARCH CENTRE BOMBAY, INDIA 1972 SPECTROIHCTOMETRIC DETERMINATION OP URANIUM IN COMMERCIAL WET PROCESS PHOSPHORIC ACID AND PHOSPHATE ROCK USING THIOCYANATE by R.A. Nagle and T.K.S. Murthy 1 . INTRODUCTION The thiocyanate method1 for the spectrophotometry determination 2 of uranium has been studied by several authors. Nietzel and DeSe3a reviewed the modifications introduced in the working of this method to make it applicable fo•r 4differen t types of samples. Clinch and Guy , Koppiker and Coworkers extracted the uranyl thiocyanate complex with tri-n-butyl phosphate (TBI?) and measured the absorbance in the organic layer. Moderate amounts of other ions could be tolerated in these procedures., During the course of studies on recovery of uranium from commercial wet process phosphoric acid the present authors felt the need for a quick and reliable spectrophotometry method. -

Aluminium in Drinking-Water
WHO/SDE/WSH/03.04/53 English only Aluminium in Drinking-water Background document for development of WHO Guidelines for Drinking-water Quality ______________________ Originally published in Guidelines for drinking-water quality, 2nd ed. Addendum to Vol. 2. Health criteria and other supporting information. World Health Organization, Geneva, 1998. © World Health Organization 2003 All rights reserved. Publications of the World Health Organization can be obtained from Marketing and Dissemination, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel: +41 22 791 2476; fax: +41 22 791 4857; email: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to Publications, at the above address (fax: +41 22 791 4806; email: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. The World Health Organization does not warrant that the information contained in this publication is complete and correct and shall not be liable for any damages incurred as a result of its use.