Polyvinyl Chloride (Pvc) Chemical Resistance Data
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

162 Part 175—Indirect Food Addi
§ 174.6 21 CFR Ch. I (4–1–19 Edition) (c) The existence in this subchapter B Subpart B—Substances for Use Only as of a regulation prescribing safe condi- Components of Adhesives tions for the use of a substance as an Sec. article or component of articles that 175.105 Adhesives. contact food shall not be construed as 175.125 Pressure-sensitive adhesives. implying that such substance may be safely used as a direct additive in food. Subpart C—Substances for Use as (d) Substances that under conditions Components of Coatings of good manufacturing practice may be 175.210 Acrylate ester copolymer coating. safely used as components of articles 175.230 Hot-melt strippable food coatings. that contact food include the fol- 175.250 Paraffin (synthetic). lowing, subject to any prescribed limi- 175.260 Partial phosphoric acid esters of pol- yester resins. tations: 175.270 Poly(vinyl fluoride) resins. (1) Substances generally recognized 175.300 Resinous and polymeric coatings. as safe in or on food. 175.320 Resinous and polymeric coatings for (2) Substances generally recognized polyolefin films. as safe for their intended use in food 175.350 Vinyl acetate/crotonic acid copoly- mer. packaging. 175.360 Vinylidene chloride copolymer coat- (3) Substances used in accordance ings for nylon film. with a prior sanction or approval. 175.365 Vinylidene chloride copolymer coat- (4) Substances permitted for use by ings for polycarbonate film. 175.380 Xylene-formaldehyde resins con- regulations in this part and parts 175, densed with 4,4′-isopropylidenediphenol- 176, 177, 178 and § 179.45 of this chapter. -

Section 2. Hazards Identification OSHA/HCS Status : This Material Is Considered Hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200)
SAFETY DATA SHEET Nonflammable Gas Mixture: Arsine / Helium / Phosgene Section 1. Identification GHS product identifier : Nonflammable Gas Mixture: Arsine / Helium / Phosgene Other means of : Not available. identification Product use : Synthetic/Analytical chemistry. SDS # : 006605 Supplier's details : Airgas USA, LLC and its affiliates 259 North Radnor-Chester Road Suite 100 Radnor, PA 19087-5283 1-610-687-5253 24-hour telephone : 1-866-734-3438 Section 2. Hazards identification OSHA/HCS status : This material is considered hazardous by the OSHA Hazard Communication Standard (29 CFR 1910.1200). Classification of the : GASES UNDER PRESSURE - Compressed gas substance or mixture ACUTE TOXICITY (inhalation) - Category 4 GHS label elements Hazard pictograms : Signal word : Warning Hazard statements : Contains gas under pressure; may explode if heated. May displace oxygen and cause rapid suffocation. Harmful if inhaled. Precautionary statements General : Read and follow all Safety Data Sheets (SDS’S) before use. Read label before use. Keep out of reach of children. If medical advice is needed, have product container or label at hand. Close valve after each use and when empty. Use equipment rated for cylinder pressure. Do not open valve until connected to equipment prepared for use. Use a back flow preventative device in the piping. Use only equipment of compatible materials of construction. Prevention : Use only outdoors or in a well-ventilated area. Avoid breathing gas. Response : IF INHALED: Remove person to fresh air and keep comfortable for breathing. Call a POISON CENTER or physician if you feel unwell. Storage : Protect from sunlight when ambient temperature exceeds 52°C/125°F. Store in a well- ventilated place. -

Gasket Chemical Services Guide
Gasket Chemical Services Guide Revision: GSG-100 6490 Rev.(AA) • The information contained herein is general in nature and recommendations are valid only for Victaulic compounds. • Gasket compatibility is dependent upon a number of factors. Suitability for a particular application must be determined by a competent individual familiar with system-specific conditions. • Victaulic offers no warranties, expressed or implied, of a product in any application. Contact your Victaulic sales representative to ensure the best gasket is selected for a particular service. Failure to follow these instructions could cause system failure, resulting in serious personal injury and property damage. Rating Code Key 1 Most Applications 2 Limited Applications 3 Restricted Applications (Nitrile) (EPDM) Grade E (Silicone) GRADE L GRADE T GRADE A GRADE V GRADE O GRADE M (Neoprene) GRADE M2 --- Insufficient Data (White Nitrile) GRADE CHP-2 (Epichlorohydrin) (Fluoroelastomer) (Fluoroelastomer) (Halogenated Butyl) (Hydrogenated Nitrile) Chemical GRADE ST / H Abietic Acid --- --- --- --- --- --- --- --- --- --- Acetaldehyde 2 3 3 3 3 --- --- 2 --- 3 Acetamide 1 1 1 1 2 --- --- 2 --- 3 Acetanilide 1 3 3 3 1 --- --- 2 --- 3 Acetic Acid, 30% 1 2 2 2 1 --- 2 1 2 3 Acetic Acid, 5% 1 2 2 2 1 --- 2 1 1 3 Acetic Acid, Glacial 1 3 3 3 3 --- 3 2 3 3 Acetic Acid, Hot, High Pressure 3 3 3 3 3 --- 3 3 3 3 Acetic Anhydride 2 3 3 3 2 --- 3 3 --- 3 Acetoacetic Acid 1 3 3 3 1 --- --- 2 --- 3 Acetone 1 3 3 3 3 --- 3 3 3 3 Acetone Cyanohydrin 1 3 3 3 1 --- --- 2 --- 3 Acetonitrile 1 3 3 3 1 --- --- --- --- 3 Acetophenetidine 3 2 2 2 3 --- --- --- --- 1 Acetophenone 1 3 3 3 3 --- 3 3 --- 3 Acetotoluidide 3 2 2 2 3 --- --- --- --- 1 Acetyl Acetone 1 3 3 3 3 --- 3 3 --- 3 The data and recommendations presented are based upon the best information available resulting from a combination of Victaulic's field experience, laboratory testing and recommendations supplied by prime producers of basic copolymer materials. -

OSHA Method 61: Phosgene
PHOSGENE - (Organic Method #61) Page 1 of 20 U.S. Department of Labor Occupational Safety & Health Administration www.osha.gov Search Advanced Search | A Technical Links > Sampling & Analytical Methods > Index PHOSGENE Method no.: 61 Matrix: Air Target concentration: 100 ppb (0.4 mg/m3) (OSHA PEL) Procedure: Air samples are collected by drawing known volumes of air through sampling tubes containing XAD-2 adsorbent which has been coated with 2 (hydroxymethyl)piperidine. The samples are desorbed with toluene and then analyzed by gas chromatography using a nitrogen selective detector. Recommended air volume and sampling rate: 240 L at 1 L/min Reliable quantitation limit: 3.5 ppb (0.014 mg/m3) Standard error of estimate at the target concentration: 6.7% (Section 4.7.) Status of method: Evaluated method. This method has been subjected to the established evaluation procedures of the Organic Methods Evaluation Branch. Date: August 1986 Chemist: Warren Hendricks Organic Methods Evaluation Branch OSHA Analytical Laboratory Salt Lake City, Utah 1. General Discussion 1.1. Background 1.1.1. History http://www.osha.gov/dts/sltc/methods/organic/org061/org061.html 7/7/2008 PHOSGENE - (Organic Method #61) Page 2 of 20 The procedures that have been used by OSHA to monitor occupational exposure to phosgene include detector tubes, monitoring dosimeters and infrared gas analyzers. None of these procedures have proven to be completely adequate for use by OSHA. These methods lack either the desired precision and accuracy or they are awkward and inconvenient for field use. This procedure was developed after it was found that phosgene would react quantitatively with 2-(hydroxymethyl)piperidine (2-HMP) to form a stable derivative, 1-aza-8-oxabicyclo[4.3.0.]inonan-9-one. -

Acetylcholinesterase: the “Hub” for Neurodegenerative Diseases And
Review biomolecules Acetylcholinesterase: The “Hub” for NeurodegenerativeReview Diseases and Chemical Weapons Acetylcholinesterase: The “Hub” for Convention Neurodegenerative Diseases and Chemical WeaponsSamir F. de A. Cavalcante Convention 1,2,3,*, Alessandro B. C. Simas 2,*, Marcos C. Barcellos 1, Victor G. M. de Oliveira 1, Roberto B. Sousa 1, Paulo A. de M. Cabral 1 and Kamil Kuča 3,*and Tanos C. C. França 3,4,* Samir F. de A. Cavalcante 1,2,3,* , Alessandro B. C. Simas 2,*, Marcos C. Barcellos 1, Victor1 Institute G. M. ofde Chemical, Oliveira Biological,1, Roberto Radiological B. Sousa and1, Paulo Nuclear A. Defense de M. Cabral (IDQBRN),1, Kamil Brazilian Kuˇca Army3,* and TanosTechnological C. C. França Center3,4,* (CTEx), Avenida das Américas 28705, Rio de Janeiro 23020-470, Brazil; [email protected] (M.C.B.); [email protected] (V.G.M.d.O.); [email protected] 1 Institute of Chemical, Biological, Radiological and Nuclear Defense (IDQBRN), Brazilian Army (R.B.S.); [email protected] (P.A.d.M.C.) Technological Center (CTEx), Avenida das Américas 28705, Rio de Janeiro 23020-470, Brazil; 2 [email protected] Mors Institute of Research (M.C.B.); on Natural [email protected] Products (IPPN), Federal (V.G.M.d.O.); University of Rio de Janeiro (UFRJ), CCS,[email protected] Bloco H, Rio de Janeiro (R.B.S.); 21941-902, [email protected] Brazil (P.A.d.M.C.) 32 DepartmentWalter Mors of Institute Chemistry, of Research Faculty of on Science, Natural Un Productsiversity (IPPN), -

Aum Shinrikyo's
Chronology of Aum Shinrikyo’s CBW Activities Introduction Six years ago, on March 20, 1995, five members of the Japanese cult Aum Shinrikyo (Supreme Truth) boarded subway trains in Tokyo, Japan, and released the deadly chemical nerve agent sarin. The attack killed 12 people and injured over 1,000, of whom 17 were critically injured (requiring intensive care), 37 were severely injured (with muscular twitching and gastrointestinal problems), and 984 were slightly injured (with pinpoint pupils but no other symptoms). Aum’s interest in chemical and biological weapons (CBW) terrorism can be traced back to 1990. Between 1990 and 1995, Aum launched 17 known CBW attacks, with motivations ranging from assassination to mass murder. Of these attacks, 10 were carried out with chemical weapons (four with sarin, four with VX, one with phosgene, and one with hydrogen cyanide) and seven attempted attacks were carried out with biological agents (four with anthrax and three with botulinum toxin, although in both cases the microbial strains were apparently nonvirulent). In addition to these cases, Aum is alleged to have killed 20 of its dissident members with VX and has been linked more tenuously to more than 19 other CBW attacks and attempted attacks (13 attacks where Aum involvement is suspected and six possible copycats). Since 1995, many of the perpetrators of the Tokyo subway attack have been jailed and are awaiting trial, and others have been sentenced to life in prison or to death by hanging. Although Aum has changed its name to Aleph, has decreased significantly in numbers, and claims to focus on its computer software company, its dangerous apocalyptic ideology remains. -

Lecture Bottle Technical Evaluation
ARCADIS 801 Corporate Center Drive Suite 300 Raleigh North Carolina 27607 MEMO Tel 919.854.1282 To: Copies: Fax 919.854.5448 Larry Daw Mary Beth Koza – UNC EHS UNC Environment, Health & Safety Alan Pinnix - ARCADIS Don Malone – ARCADIS Jim Shilliday – ARCADIS Ruddie Clarkson - ARCADIS From: David Proffitt Date: ARCADIS Project No.: June 27, 2008 NC000239.0017 Subject: UNC Airport Road Waste Disposal Area Lecture Bottle Technical Evaluation Due to the recent discovery of small compressed gas cylinders at the UNC Airport Road Waste Disposal Area, ARCADIS was asked to re-evaluate the site remediation practices and engineering controls to help minimize the potential for impacts to site workers and the surrounding community, including personnel and animals at the Orange County Animal Shelter. To facilitate this additional evaluation ARCADIS integrated an air monitoring and sampling expert into the project team to review the site practices and conditions. The overall project team reviewed all aspects of the recent cylinder event including discovery, intermediate handling, over-pack application, and finally shipping from the site. Several aspects were noted as having a higher potential to greatly affect capture and dispersion of an emission event from a cylinder. These aspects included: 1- The embossed number on one of the cylinders suggests it could have originally contained one of the following compounds: phosphine, arsine, diborane, nitric oxide, and nitrogen dioxide. The color of an additional cylinder suggests that it could potentially contain phosgene. The evaluation discussed in this memo used arsine and phosgene as a worst-case condition model. 2 - The arrangement of the pit and the tent-like enclosure over the work area allows direct wind only from its open ends. -

Concept for CBRN Full Facepiece Air Purifying Respirator Standard
June 15, 2002 (DRAFT FOR DISCUSSION) Concept for CBRN Full Facepiece Air Purifying Respirator Standard (1) Goal: Develop a NIOSH, NPPTL, tight fitting, full facepiece, air purifying respirator standard that addresses CBRN materials identified as inhalation hazards and/or possible terrorist hazards using a minimum number of filters for emergency responders. Target: Four (4) filters Short Duration Long Duration TIMs 15 minutes* 60 minutes* TIMs plus CO 15 minutes* 60 minutes* * Indicated times are for illustration only. Actual times will be established from hazard modeling and developmental test results. (2) Hazards: NIOSH has been evaluating various lists of chemicals that could be deployed as a result of a terrorist incident. In an effort to reduce the number of certification tests necessary as part of a Chemical Biological Radiological Nuclear (CBRN) Air-Purifying Respirator (APR) standard, efforts have been underway to categorize potential respiratory hazards into families with a representative test chemical identified for each family. The following information is a synopsis of this effort to date. The current carbon technology used in canisters and cartridges were reviewed from existing certification standards. The current standards for gas masks in Europe and the U.S. (NIOSH) were reviewed. The military purchasing specification for ASZM-T carbon for C2A1 military canisters was also reviewed. The most common parameters identified from the review of the military specification and the certification standards were the middle range certification challenges. Some of the test chemicals were considered to be redundant, since other test chemicals would guarantee the carbon effectiveness against the chemicals in question (Chlorine, Hydrogen Chloride, Hydrogen Fluoride, Phosphine, CS & CN Tear Gases). -

Website Chlorine Pwdr Sugar Water Phosgene Tear
Phosgene 2 Gases 44P make Hydrogen website Tear Gas,2 KCN 2, make somoom Chemical Agents Chlorine pwdr Ricin CHLORINE,2 Sugar water Phosgene Phosgene, also known as carbonic dichloride, colorless, extremely toxic gas of formula COCl2 with an unpleasant, irritating odor at high concentrations. It is prepared by the reaction of carbon monoxide with chlorine in the presence of a catalyst. Phosgene is poisonous in concentrations above 50 parts per million of air, and if inhaled, it causes severe and often fatal edema of the lungs within a few hours. It was used in World War I as a poison gas but today is used principally as an intermediate in the synthesis of organic compounds, including carbonic esters, isocyanates, polyurethanes, and dyes. Phosgene is 3.43 times heavier than air; the gas melts at -118° C (-180.4° F) and boils at 8.3° C (46.9° F). Look here Chemical war section. Tear Gas I INTRODUCTION Tear Gas, chemical substance that produces a primary physical effect of stinging or tearing eyes. Tear gas also irritates other mucous membranes and causes choking and coughing. People exposed to higher concentrations may experience burning, itching, or even blistering skin. As a form of riot control, police often use clouds of tear gas to break up crowds of people. A rifle-fired grenade or a thrown canister usually delivers tear gas, but smaller hand- held spray devices also exist. Tear gas may also be used to force the surrender of fugitives hiding in a building. Dogs and horses are relatively unaffected by tear gas, so they can add to the riot-control effect of the gas. -

Chemical Weapons Technology Section 4—Chemical Weapons Technology
SECTION IV CHEMICAL WEAPONS TECHNOLOGY SECTION 4—CHEMICAL WEAPONS TECHNOLOGY Scope Highlights 4.1 Chemical Material Production ........................................................II-4-8 4.2 Dissemination, Dispersion, and Weapons Testing ..........................II-4-22 • Chemical weapons (CW) are relatively inexpensive to produce. 4.3 Detection, Warning, and Identification...........................................II-4-27 • CW can affect opposing forces without damaging infrastructure. 4.4 Chemical Defense Systems ............................................................II-4-34 • CW can be psychologically devastating. • Blister agents create casualties requiring attention and inhibiting BACKGROUND force efficiency. • Defensive measures can be taken to negate the effect of CW. Chemical weapons are defined as weapons using the toxic properties of chemi- • Donning of protective gear reduces combat efficiency of troops. cal substances rather than their explosive properties to produce physical or physiologi- • Key to employment is dissemination and dispersion of agents. cal effects on an enemy. Although instances of what might be styled as chemical weapons date to antiquity, much of the lore of chemical weapons as viewed today has • CW are highly susceptible to environmental effects (temperature, its origins in World War I. During that conflict “gas” (actually an aerosol or vapor) winds). was used effectively on numerous occasions by both sides to alter the outcome of • Offensive use of CW complicates command and control and battles. A significant number of battlefield casualties were sustained. The Geneva logistics problems. Protocol, prohibiting use of chemical weapons in warfare, was signed in 1925. Sev- eral nations, the United States included, signed with a reservation forswearing only the first use of the weapons and reserved the right to retaliate in kind if chemical weapons were used against them. -
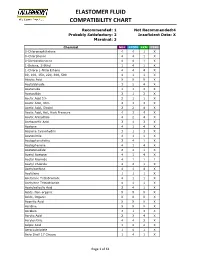
Elastomer Fluid Compatibility Chart
ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE 0-Chloronaphthalene 4 4 1 X 0-Chlorphenol 4 4 1 X 0-Dichlorobenzene 4 4 1 X 1-Butene, 2-Ethyl 1 4 1 X 1-Chloro 1-Nitro Ethane 4 4 4 X 90, 100, 150, 220, 300, 500 4 1 1 X Abietic Acid X X X X Acetaldehyde 3 2 4 X Acetamide 1 1 3 X Acetanilide 3 1 3 X Acetic Acid 5% 2 1 1 X Acetic Acid, 30% 2 1 3 X Acetic Acid, Glacial 2 2 4 X Acetic Acid, Hot, High Pressure 4 3 4 X Acetic Anhydride 4 2 4 X Acetoacetic Acid 3 1 3 X Acetone 4 1 4 X Acetone Cyanohydrin 3 1 3 X Acetonitrile 3 1 1 X Acetophenetidine 2 4 1 X Acetophenone 4 1 4 X Acetotoluidide 2 4 1 X Acetyl Acetone 4 1 4 X Acetyl Bromide 4 1 1 1 Acetyl Chloride 4 4 1 X Acetylacetone 4 1 4 X Acetylene 1 1 1 X Acetylene Tetrabromide 4 1 1 X Acetylene Tetrachloride 4 1 1 X Acetylsalicylic Acid 2 4 1 X Acids, Non-organic X X X X Acids, Organic X X X X Aconitic Acid X X X X Acridine X X X X Acrolein 3 1 3 X Acrylic Acid 2 3 4 X Acrylonitrile 4 4 3 X Adipic Acid 1 2 2 X Aero Lubriplate 1 4 1 X Aero Shell 17 Grease 1 4 1 X Page 1 of 61 ELASTOMER FLUID COMPATIBILITY CHART Recommended: 1 Not Recommended:4 Probably Satisfactory: 2 Insuficient Data: X Marginal: 3 Chemical NBR EPDM FKM PTFE Aero Shell 1AC Grease 1 4 1 X Aero Shell 750 2 4 1 X Aero Shell 7A Grease 1 4 1 X Aerosafe 2300 4 1 4 X Aerosafe 2300W 4 1 4 X Aerozene 50 (50% Hydrazine 50% UDMH) 3 1 4 X Air, 1 1 1 X Air, 200 - 300° F 3 2 1 X Air, 300 - 400° F 4 4 1 X Air, 400 - 500° F 4 4 3 X Air, -

Oxides Affording Phosphines(III) and Their Metal Catalysts
pubs.acs.org/Organometallics Article A Mild One-Pot Reduction of Phosphine(V) Oxides Affording Phosphines(III) and Their Metal Catalysts Łukasz Kapusniak,́ Philipp N. Plessow, Damian Trzybinski,́ Krzysztof Wozniak,́ Peter Hofmann, and Phillip Iain Jolly* Cite This: Organometallics 2021, 40, 693−701 Read Online ACCESS Metrics & More Article Recommendations *sı Supporting Information ABSTRACT: The metal-free reduction of a range of phosphine(V) oxides employing oxalyl chloride as an activating agent and hexachlorodisilane as reducing reagent has been achieved under mild reaction conditions. The method was successfully applied to the reduction of industrial waste byproduct triphenylphosphine(V) oxide, closing the phosphorus cycle to cleanly regenerate triphenylphosphine(III). Mechanistic studies and quantum chemical calculations support the attack of the dissociated chloride anion of intermediated phosphonium salt at the silicon of the disilane as the rate-limiting step for deprotection. The exquisite purity of the resultant phosphine(III) ligands after the simple removal of volatiles under reduced pressure circumvents laborious purification prior to metalation and has permitted the facile formation of important transition metal catalysts. ■ INTRODUCTION Scheme 1. Phosphine Synthesis: Background and This a Applications of Phosphine(III) Ligands and Synthesis. Work Phosphines and their derivatives are of significant importance to both academic and industrial chemistry. In particular, within organic chemistry phosphine(III) compounds have a distin- guished history, mediating classical transformations such as the Appel,1 Mitsunobu,2 and Wittig3,4 reactions. Additionally, the ready modulation of electronic and steric properties of phosphine(III) has made them excellent ligands for the formation of well-defined transition metal complexes,5 although recalcitrant phosphine(V) oxides arise, when 6 Downloaded via KIT BIBLIOTHEK on April 16, 2021 at 16:05:05 (UTC).