Review Reports Is a Part of That Process
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Lifetime Organophosphorous Insecticide Use Among Private Pesticide Applicators in the Agricultural Health Study
Journal of Exposure Science and Environmental Epidemiology (2012) 22, 584 -- 592 & 2012 Nature America, Inc. All rights reserved 1559-0631/12 www.nature.com/jes ORIGINAL ARTICLE Lifetime organophosphorous insecticide use among private pesticide applicators in the Agricultural Health Study Jane A. Hoppin1, Stuart Long2, David M. Umbach3, Jay H. Lubin4, Sarah E. Starks5, Fred Gerr5, Kent Thomas6, Cynthia J. Hines7, Scott Weichenthal8, Freya Kamel1, Stella Koutros9, Michael Alavanja9, Laura E. Beane Freeman9 and Dale P. Sandler1 Organophosphorous insecticides (OPs) are the most commonly used insecticides in US agriculture, but little information is available regarding specific OP use by individual farmers. We describe OP use for licensed private pesticide applicators from Iowa and North Carolina in the Agricultural Health Study (AHS) using lifetime pesticide use data from 701 randomly selected male participants collected at three time periods. Of 27 OPs studied, 20 were used by 41%. Overall, 95% had ever applied at least one OP. The median number of different OPs used was 4 (maximum ¼ 13). Malathion was the most commonly used OP (74%) followed by chlorpyrifos (54%). OP use declined over time. At the first interview (1993--1997), 68% of participants had applied OPs in the past year; by the last interview (2005--2007), only 42% had. Similarly, median annual application days of OPs declined from 13.5 to 6 days. Although OP use was common, the specific OPs used varied by state, time period, and individual. Much of the variability in OP use was associated with the choice of OP, rather than the frequency or duration of application. -

Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia Coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface
Biotechnol. Bioprocess Eng. 2000, 5: 436-440 Biodetoxification of Coumaphos Insecticide Using Immobilized Escherichia coli Expressing Organophosphorus Hydrolase Enzyme on Cell Surface Ayman H. Mansee2, Wilfred Chen1, and Ashok Mulchandani1* 1 Department of Chemical and Environmental Engineering, University of California, Riverside, CA 92521, USA 2 On leave from Pesticide Chemistry Department, Faculty of Agriculture (El-Shatby), Alexandria University, Alexandria, Egypt Abstract Recently, we reported an improved technology for the degradation of organophos- phate nerve agents using whole cells of genetically engineered Escherichia coli that anchored and displayed the enzyme organophosphorus hydrolase on the cell surface. In this paper we report the immobilization of these cells on highly porous sintered glass beads and the subse- quent application of the immobilized cell in a continuous-flow packed bed bioreactor for the biodetoxification of a widely used insecticide, coumaphos. Keywords: OPH, pesticides, nerve agents, bioreactor, porous sintered glass beads INTRODUCTION (APHIS) operates a Tick Eradication Program designed to prevent the re-introduction of Cattle Fever into the Neurotoxic organophosphates (OPs), amongst the United States through ticks (Boophilus microplus and most toxic compound known, are widely used as pesti- Boophilus annulatus) on cattle imported from Mexico. cides, insecticides and chemical warfare agents. In the The primary tool used in the eradication program is a United States annually over 40 million kg of organo- series of dipping vats placed at the border crossing phosphate pesticides are consumed [1] while another 20 points. Cattle coming in from Mexico must be dipped million kg are produced for export [2]. Chlorpyrifos in vats before entering the US. Currently, coumaphos is (lorsban) and parathion are two of most popular pesti- the pesticide of choice for this application. -

Recent Advances on Detection of Insecticides Using Optical Sensors
sensors Review Recent Advances on Detection of Insecticides Using Optical Sensors Nurul Illya Muhamad Fauzi 1, Yap Wing Fen 1,2,*, Nur Alia Sheh Omar 1,2 and Hazwani Suhaila Hashim 2 1 Functional Devices Laboratory, Institute of Advanced Technology, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] (N.I.M.F.); [email protected] (N.A.S.O.) 2 Department of Physics, Faculty of Science, Universiti Putra Malaysia, Serdang 43400, Selangor, Malaysia; [email protected] * Correspondence: [email protected] Abstract: Insecticides are enormously important to industry requirements and market demands in agriculture. Despite their usefulness, these insecticides can pose a dangerous risk to the safety of food, environment and all living things through various mechanisms of action. Concern about the environmental impact of repeated use of insecticides has prompted many researchers to develop rapid, economical, uncomplicated and user-friendly analytical method for the detection of insecticides. In this regards, optical sensors are considered as favorable methods for insecticides analysis because of their special features including rapid detection time, low cost, easy to use and high selectivity and sensitivity. In this review, current progresses of incorporation between recognition elements and optical sensors for insecticide detection are discussed and evaluated well, by categorizing it based on insecticide chemical classes, including the range of detection and limit of detection. Additionally, this review aims to provide powerful insights to researchers for the future development of optical sensors in the detection of insecticides. Citation: Fauzi, N.I.M.; Fen, Y.W.; Omar, N.A.S.; Hashim, H.S. Recent Keywords: insecticides; optical sensor; recognition element Advances on Detection of Insecticides Using Optical Sensors. -
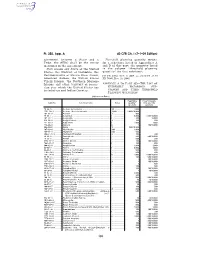
40 CFR Ch. I (7–1–09 Edition) Pt. 355, App. A
Pt. 355, App. A 40 CFR Ch. I (7–1–09 Edition) agreement between a State and a Threshold planning quantity means, Tribe, the SERC shall be the entity for a substance listed in Appendices A identified in the agreement. and B of this part, the quantity listed State means any State of the United in the column ‘‘threshold planning States, the District of Columbia, the quantity’’ for that substance. Commonwealth of Puerto Rico, Guam, [73 FR 65462, Nov. 3, 2008, as amended at 73 American Samoa, the United States FR 76960, Dec. 18, 2008] Virgin Islands, the Northern Mariana Islands, any other territory or posses- APPENDIX A TO PART 355—THE LIST OF sion over which the United States has EXTREMELY HAZARDOUS SUB- jurisdiction and Indian Country. STANCES AND THEIR THRESHOLD PLANNING QUANTITIES [Alphabetical Order] Reportable Threshold plan- CAS No. Chemical name Notes quantity * ning quantity (pounds) (pounds) 75–86–5 ................ Acetone Cyanohydrin ................................................. 10 ................ 1,000 1752–30–3 ............ Acetone Thiosemicarbazide ....................................... 1,000 ........... 1,000/10,000 107–02–8 .............. Acrolein ....................................................................... 1 .................. 500 79–06–1 ................ Acrylamide .................................................................. f ................... 5,000 1,000/10,000 107–13–1 .............. Acrylonitrile ................................................................. f ................... 100 10,000 -

NMP-Free Formulations of Neonicotinoids
(19) & (11) EP 2 266 400 A1 (12) EUROPEAN PATENT APPLICATION (43) Date of publication: (51) Int Cl.: 29.12.2010 Bulletin 2010/52 A01N 43/40 (2006.01) A01N 43/86 (2006.01) A01N 47/40 (2006.01) A01N 51/00 (2006.01) (2006.01) (2006.01) (21) Application number: 09305544.0 A01P 7/00 A01N 25/02 (22) Date of filing: 15.06.2009 (84) Designated Contracting States: (72) Inventors: AT BE BG CH CY CZ DE DK EE ES FI FR GB GR • Gasse, Jean-Jacques HR HU IE IS IT LI LT LU LV MC MK MT NL NO PL 27600 Saint-Aubin-Sur-Gaillon (FR) PT RO SE SI SK TR • Duchamp, Guillaume Designated Extension States: 92230 Gennevilliers (FR) AL BA RS • Cantero, Maria 92230 Gennevilliers (FR) (71) Applicant: NUFARM 92233 Gennevelliers (FR) (74) Representative: Cabinet Plasseraud 52, rue de la Victoire 75440 Paris Cedex 09 (FR) (54) NMP-free formulations of neonicotinoids (57) The invention relates to NMP-free liquid formulation comprising at least one nicotinoid and at least one aprotic polar component selected from the group comprising the compounds of formula I, II or III below, and mixtures thereof, wherein R1 and R2 independently represent H or an alkyl group having less than 5 carbons, preferably a methyl group, and n represents an integer ranging from 0 to 5, and to their applications. EP 2 266 400 A1 Printed by Jouve, 75001 PARIS (FR) EP 2 266 400 A1 Description Technical Field of the invention 5 [0001] The invention relates to novel liquid formulations of neonicotinoids and to their use for treating plants, for protecting plants from pests and/or for controlling pests infestation. -

Mechanisms of Differential Toxicity Between Honey Bee (<I>Apis
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Dissertations and Student Research in Entomology Entomology, Department of 5-2014 Mechanisms of differential toxicity between honey bee (Apis mellifera L.) castes with an emphasis on coumaphos Lizette Dahlgren University of Nebraska-Lincoln Follow this and additional works at: http://digitalcommons.unl.edu/entomologydiss Part of the Entomology Commons Dahlgren, Lizette, "Mechanisms of differential toxicity between honey bee (Apis mellifera L.) castes with an emphasis on coumaphos" (2014). Dissertations and Student Research in Entomology. 32. http://digitalcommons.unl.edu/entomologydiss/32 This Article is brought to you for free and open access by the Entomology, Department of at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Dissertations and Student Research in Entomology by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Mechanisms of differential toxicity between honey bee (Apis mellifera L.) castes with an emphasis on coumaphos by Lizette A. P. Dahlgren A DISSERTATION Presented to the Faculty of The Graduate College at the University of Nebraska In Partial Fulfillment For the Degree of Doctor of Philosophy Major: Entomology Under the Supervision of Professors Marion Ellis & Blair Siegfried Lincoln, Nebraska May, 2014 Mechanisms of differential toxin sensitivity between honey bee (Apis mellifera L.) castes with an emphasis on coumaphos Lizette Dahlgren, Ph.D. University of Nebraska, 2014 Advisors: Marion Ellis & Blair Siegfried Abstract: Acaricides are used to treat honey bee (Apis mellifera L.) colonies to control the parasitic Varroa mite (Varroa destructor Anderson & Trueman), a worldwide threat to honey bee health. As such, these compounds act as drugs to mitigate bee losses but may also stress the bees. -

The Hazardous Substances and Waste Dangerous Goods Regulations
1 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 The Hazardous Substances and Waste Dangerous Goods Regulations being Chapter E-10.2 Reg 3 (effective April 1, 1989) as amended by Saskatchewan Regulations 25/92, 107/92, 28/94, 3/95 and 63/2000. NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 HAZARDOUS SUBSTANCES AND E-10.2 REG 3 WASTE DANGEROUS GOODS Table of Contents 1 Title 11 Decision to grant approval INTERPRETATION 12 Approval not assignable, exception 2 Interpretation 13 Duties of operator, owner HAZARDOUS SUBSTANCES 14 Prohibition re storage in above-ground tanks 3 Designation of hazardous substances 15 Prohibition re storage in underground tanks 16 Prohibition re storage in certain containers or DESIGNATION AS HAZARDOUS WASTES stockpiles 3.1 Designation of waste dangerous goods 17 Decommissioning as hazardous wastes TRANSFERAL OF WASTE DANGEROUS GOODS CHARACTERIZATION OF SUBSTANCES 18 Transferal of waste dangerous goods 4 Characteristics of certain hazardous substances EXEMPTION FROM REQUIREMENTS Appendix A 5 General Exemptions INDUSTRIAL HAZARDOUS SUBSTANCES 6 Underground storage facilities Appendix B 7 Above-ground storage facilities ACUTE HAZARDOUS SUBSTANCES 8 Storage in small containers Appendix C APPROVAL TO STORE ENVIRONMENTAL PERSISTENT OR 9 Approval to store CHRONIC HAZARDOUS SUBSTANCES APPROVAL TO CONSTRUCT Appendix D 10 Approval to construct WASTE DANGEROUS GOODS 3 HAZARDOUS SUBSTANCES AND WASTE DANGEROUS GOODS E-10.2 REG 3 CHAPTER E-10.2 REG 3 The Environmental Management and Protection Act Title 1 These regulations may be cited as The Hazardous Substances and Waste Dangerous Goods Regulations. -

US Environmental Protection Agency Office of Pesticide Programs
US Environmental Protection Agency Office of Pesticide Programs Reregistration Eligibility Decision for Coumaphos When EPA concluded the organophosphate (OP) cumulative risk assessment in July 2006, all tolerance reassessment and reregistration eligibility decisions for individual OP pesticides were considered complete. OP Interim Reregistration Eligibility Decisions (IREDs), therefore, are considered completed REDs. OP tolerance reassessment decisions (TREDs) also are considered completed. Combined PDF document consists of the following: • Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides (July 31, 2006) • Coumaphos TRED UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative risks -

Environmental Protection Agency Pt. 355, App. A
Environmental Protection Agency Pt. 355, App. A Release means any spilling, leaking, the facility is located. In the absence pumping, pouring, emitting, emptying, of a SERC for a State or Indian Tribe, discharging, injecting, escaping, leach- the Governor or the chief executive of- ing, dumping, or disposing into the en- ficer of the tribe, respectively, shall be vironment (including the abandonment the SERC. Where there is a cooperative or discarding of barrels, containers, agreement between a State and a and other closed receptacles) of any Tribe, the SERC shall be the entity hazardous chemical, EHS, or CERCLA identified in the agreement. hazardous substance. Solution means any aqueous or or- Reportable quantity means, for any ganic solutions, slurries, viscous solu- CERCLA hazardous substance, the tions, suspensions, emulsions, or quantity established in Table 302.4 of 40 pastes. CFR 302.4, for such substance. For any State means any State of the United EHS, reportable quantity means the States, the District of Columbia, the quantity established in Appendices A Commonwealth of Puerto Rico, Guam, and B of this part for such substance. American Samoa, the United States Unless and until superseded by regula- Virgin Islands, the Northern Mariana tions establishing a reportable quan- Islands, any other territory or posses- tity for newly listed EHSs or CERCLA sion over which the United States has hazardous substances, a weight of 1 jurisdiction and Indian Country. pound shall be the reportable quantity. Threshold planning quantity means, SERC means the State Emergency for a substance listed in Appendices A Response Commission for the State in and B of this part, the quantity listed which the facility is located except in the column ‘‘threshold planning where the facility is located in Indian quantity’’ for that substance. -

The Translation of Insecticide Resistance in Drosophila Hydei from Organophosphates to Carbamates Chandler Witt 2016-2017 Increase in Insecticide Resistance
The Translation of Insecticide Resistance in Drosophila Hydei from Organophosphates to Carbamates Chandler Witt 2016-2017 Increase in Insecticide Resistance ● There was, as of 1990, Insecticide resistance recorded in at least 504 species of insects and mites ● There was a 13% growth in resistance from 1984 to 1990 ● 60 countries have reported some level of resistance to one regularly used insecticide ● 49 of those 60 have reported resistance to two or more of those insecticides Translation of Insecticide Resistance ● Is the ability for a population resistant to one specific type of insecticide to gain resistant to another. ● Has been observed within insecticide classes ● Department of Entomology found that a point mutation in the gene coding for the GABAA neurotransmitter can grant Drosophila high levels of resistance to Picrotoxinin and Cyclodiene Conditioned Resistance ● Conditioned resistance is the gradual gain of resistance in a population ● This is most common through gradual exposure, low levels of a stressor ● Generally takes longer to develop in the wild Naled/Dibrom ● Organophosphate ● Restricted use insecticide ● Used due to a fast knockdown ● Limited to no environmental persistence ● Due to its fast knockdown rate and short persistence, there is limited resistance to it. ● Acts by interfering with the activities of the enzyme cholinesterase Coumaphos ● Organophosphate ● General use insecticide ● Used in a dust ● Targets mites, flies, ticks and fleas ● Highly toxic to all animals ● There is resistance ● Acts by interfering with the activities of the enzyme cholinesterase Bendiocarb ● Carbamate ● Both restricted and General use ● Targets most insects, specifically household Pests, as well as snails and slugs ● Highly toxic to all animals ● There is resistance ● Acts by interfering with the activities of the enzyme cholinesterase Materials ● Organophosphate Naled, now called Dibrom, Analytical Standard ● Organophosphate Coumaphos, found in 1% concentration in CoRal Livestock Dust. -

Pesticides Were Considered Complete
US Environmental Protection Agency Office of Pesticide Programs Reregistration Eligibility Decision for Azinphos-Methyl When EPA concluded the organophosphate (OP) cumulative risk assessment in July 2006, all tolerance reassessment and reregistration eligibility decisions for individual OP pesticides were considered complete. OP Interim Reregistration Eligibility Decisions (IREDs), therefore, are considered completed REDs. OP tolerance reassessment decisions (TREDs) also are considered completed. Combined PDF document consists of the following: • Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides (July 31, 2006) • Azinphos-Methyl IRED UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON D.C., 20460 OFFICE OF PREVENTION, PESTICIDES AND TOXIC SUBSTANCES MEMORANDUM DATE: July 31, 2006 SUBJECT: Finalization of Interim Reregistration Eligibility Decisions (IREDs) and Interim Tolerance Reassessment and Risk Management Decisions (TREDs) for the Organophosphate Pesticides, and Completion of the Tolerance Reassessment and Reregistration Eligibility Process for the Organophosphate Pesticides FROM: Debra Edwards, Director Special Review and Reregistration Division Office of Pesticide Programs TO: Jim Jones, Director Office of Pesticide Programs As you know, EPA has completed its assessment of the cumulative -

Call for Information on Substances Containing Organophosphates and Carbamates (Opcs)
Call for information on substances containing organophosphates and carbamates (OPCs) Summary The Environmental Protection Authority (EPA) is seeking information to inform a potential reassessment of substances containing organophosphates and carbamates (OPCs) used as active ingredients in veterinary medicines or pesticides. This call for information relates mainly to a set of substances that were not included in the EPA’s previous organophosphate and carbamate reassessments, which were completed in June 2013 (APP201045 – OPC plant protection insecticides), September 2015 (APP202097 - dichlorvos-containing substances) and January 2016 (APP202098 – carbaryl, chlorpyrifos and diazinon substances used for purposes other than plant protection). The focus of this call for information is the remaining approved substances containing bendiocarb, carbaryl, chlorfenvinphos, coumaphos, fenitrothion, fenthion, formetanate, furathiocarb, maldison/malathion, methiocarb, methomyl, propetamphos, propoxur, temephos, tetrachlorvinphos, or thiodicarb as the active ingredient. A full list of substance approvals we are interested in is in Tables 1-6. In order to understand the current OPC landscape, the EPA is seeking information whether these substances are still in use in New Zealand, and if so, to what extent. We believe a number of these OPCs are no longer being used. Where the use of an OPC-containing substance is declining, we are seeking information on this reduction of use, and any critical uses or benefits that illustrate the positive effects of those approvals which still remain in use. Additionally, we would like to know if any alternative substances can replace these declining-use OPC approvals. Please provide your feedback to the EPA by 5pm, 28 May 2021. Background information The EPA regulates hazardous substances (chemicals and chemical mixtures) under the Hazardous Substances and New Organisms (HSNO) Act.