The Foot of Homo Floresiensis
Total Page:16
File Type:pdf, Size:1020Kb
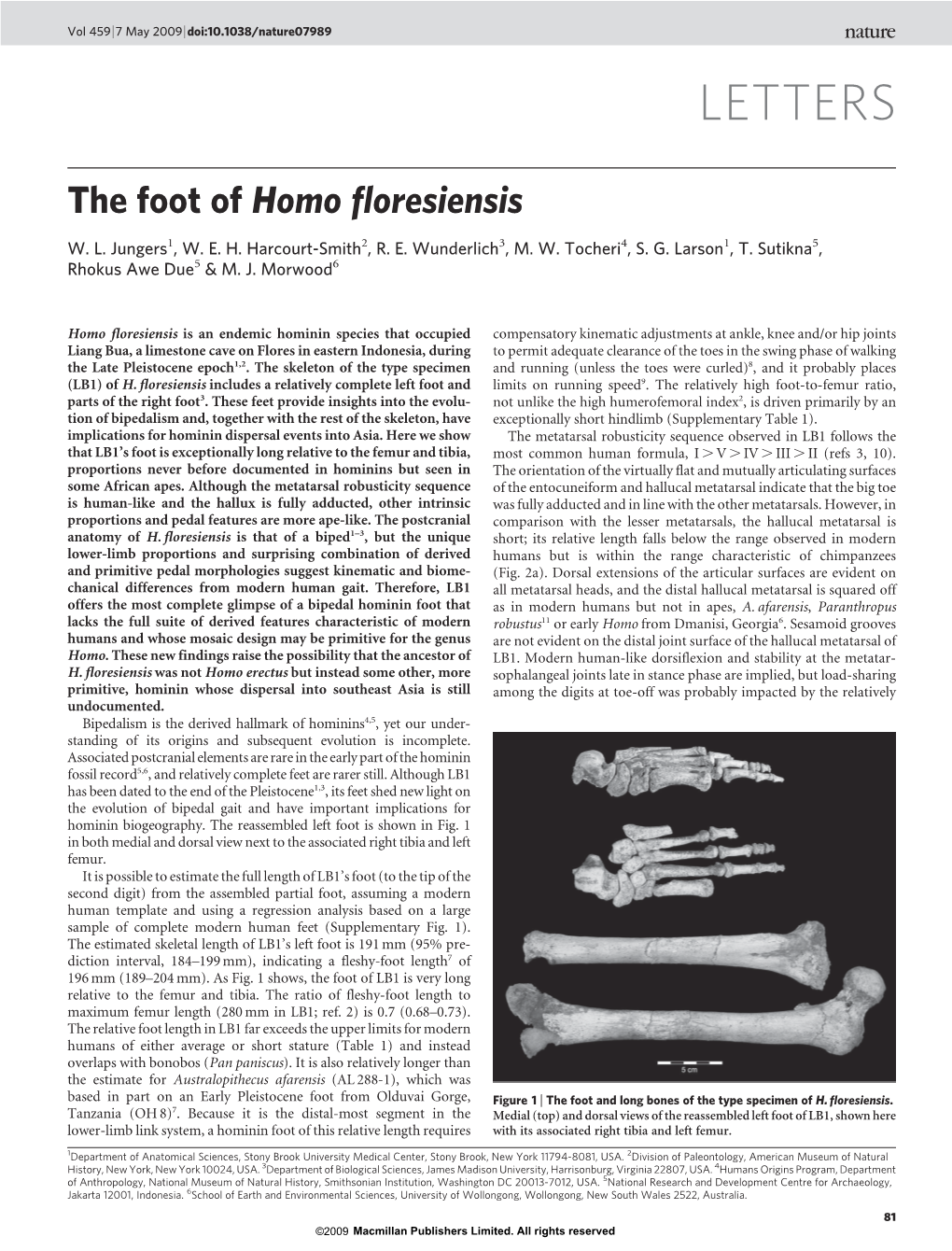
Load more
Recommended publications
-

Darwin and the Recent African Origin of Modern Humans
EDITORIAL Darwin and the recent African origin of modern humans Richard G. Klein1 Program in Human Biology, Stanford University, Stanford, CA 94305 n this 200th anniversary of When Darwin and Huxley were ac- The Course of Human Evolution Charles Darwin’s birth and tive, many respected scientists sub- In the absence of fossils, Darwin could the 150th anniversary of the scribed to the now discredited idea that not have predicted the fundamental pat- publication of his monumen- human races represented variably tern of human evolution, but his evolu- Otal The Origin of Species (1859) (1), it evolved populations of Homo sapiens. tionary theory readily accommodates seems fitting to summarize Darwin’s The original Neanderthal skull had a the pattern we now recognize. Probably views on human evolution and to show conspicuous browridge, and compared the most fundamental finding is that the how far we have come since. Darwin with the skulls of modern humans, it australopithecines, who existed from at famously neglected the subject in The was decidedly long and low. At the same least 4.5 million to 2 million years ago, Origin, except near the end where he time, it had a large braincase, and Hux- were distinguished from apes primarily noted only that ‘‘light would be thrown ley regarded it as ‘‘the extreme term of by anatomical specializations for habit- on the origin of man and his history’’ by a series leading gradually from it to the ual bipedalism, and it was only after 2 the massive evidence he had compiled highest and best developed of [modern] million years ago that people began to for evolution by means of natural selec- human crania.’’ It was only in 1891 that acquire the other traits, including our tion. -

13.10 News 934-935
NEWS NATURE|Vol 437|13 October 2005 Forbidden cave: without permits, the discoverers of the hobbit are unable to continue digging at Liang Bua cave. C. TURNEY, UNIV. WOLLONGONG UNIV. TURNEY, C. More evidence for hobbit unearthed as diggers are refused access to cave Even as researchers uncover more details The study of the bones that have so far been about the ancient ‘hobbit’ people of Indonesia, recovered has already been hindered, the they fear that they may never return to Liang researchers complain. Last winter, after hold- Bua cave, where the crucial specimens were ing the remains for about four months, Jacob found in 2003. returned them to the discovery team with “My guess is that we will not work at Liang some bones broken or shattered. The bones Bua again, this year or any other year,” says may have been damaged when casts were team leader Michael Morwood, an archaeolo- attempted or during transport. Today’s article C. TURNEY, UNIV. WOLLONGONG UNIV. TURNEY, C. gist at the University of New England in was delayed by at least six months, the authors Armidale. say, because the bones were not available The latest findings from the cave reveal, for follow-up studies. The newly described among other details, that the metre-tall jaw, for instance, was broken in half between humans, known as Homo floresiensis, lived on the front teeth, and the break has obliterated the island of Flores as little as 12,000 years ago certain key structures. (see page 1012). But continued exploration at “It’s an outrage,” says team anthropologist Liang Bua is being blocked, the researchers say, Peter Brown, also based at the University of because the discovery of miniature humans New England. -

© in This Web Service Cambridge University
Cambridge University Press 978-1-107-01829-7 - Human Adaptation in the Asian Palaeolithic: Hominin Dispersal and Behaviour during the Late Quaternary Ryan J. Rabett Index More information Index Abdur, 88 Arborophilia sp., 219 Abri Pataud, 76 Arctictis binturong, 218, 229, 230, 231, 263 Accipiter trivirgatus,cf.,219 Arctogalidia trivirgata, 229 Acclimatization, 2, 7, 268, 271 Arctonyx collaris, 241 Acculturation, 70, 279, 288 Arcy-sur-Cure, 75 Acheulean, 26, 27, 28, 29, 45, 47, 48, 51, 52, 58, 88 Arius sp., 219 Acheulo-Yabrudian, 48 Asian leaf turtle. See Cyclemys dentata Adaptation Asian soft-shell turtle. See Amyda cartilaginea high frequency processes, 286 Asian wild dog. See Cuon alipinus hominin adaptive trajectories, 7, 267, 268 Assamese macaque. See Macaca assamensis low frequency processes, 286–287 Athapaskan, 278 tropical foragers (Southeast Asia), 283 Atlantic thermohaline circulation (THC), 23–24 Variability selection hypothesis, 285–286 Attirampakkam, 106 Additive strategies Aurignacian, 69, 71, 72, 73, 76, 78, 102, 103, 268, 272 economic, 274, 280. See Strategy-switching Developed-, 280 (economic) Proto-, 70, 78 technological, 165, 206, 283, 289 Australo-Melanesian population, 109, 116 Agassi, Lake, 285 Australopithecines (robust), 286 Ahmarian, 80 Azilian, 74 Ailuropoda melanoleuca fovealis, 35 Airstrip Mound site, 136 Bacsonian, 188, 192, 194 Altai Mountains, 50, 51, 94, 103 Balobok rock-shelter, 159 Altamira, 73 Ban Don Mun, 54 Amyda cartilaginea, 218, 230 Ban Lum Khao, 164, 165 Amyda sp., 37 Ban Mae Tha, 54 Anderson, D.D., 111, 201 Ban Rai, 203 Anorrhinus galeritus, 219 Banteng. See Bos cf. javanicus Anthracoceros coronatus, 219 Banyan Valley Cave, 201 Anthracoceros malayanus, 219 Barranco Leon,´ 29 Anthropocene, 8, 9, 274, 286, 289 BAT 1, 173, 174 Aq Kupruk, 104, 105 BAT 2, 173 Arboreal-adapted taxa, 96, 110, 111, 113, 122, 151, 152, Bat hawk. -

Test 3 Study Guide
Test 3 Study Guide ANATOMICALLY MODERN HUMANS- earliest fossils found in Africa dated to about 200,000 years ago, well-rounded rear of skull (no occipital bun), high skull (doesn’t slope), small brow ridges (supra orbital torus), noticeable chin, associated with Upper Paleolithic tools (some had blades and some were made of bone), created shelters and they were the first to create artistic objects (note cave drawings possible sympathetic magic-related to the desire to capture more animals). Omo- oldest known AMH found at Omo site in Ethiopia—date ~ 195,000ya. Same morphology as noted above. homo heidelbergensis- a species of archaic human w/ a brain size close to that of modern humans (~1500-1800cc’s or more) but had a larger face and lived in Africa, Europe and Asia between 800,000 and 200,000 ya. Cro-Magnon Man- found in Cro-Magnon, France…dates between 27,000-23,000ya, earliest AMH populations found in Europe, very sophisticated, fished, cured ailments, made clothing and jewelry, built rafts etc… complication: Homo Floresiensis “hobbit” Discovered in Liang Bua cave on the island of Flores in Indonesia, 3.5”, 417cc’s found w/ tools and bones, dates between 12,000 and 94,000 ya, possible dwarf species, AMH characteristics. Possible explanations= island dwarfism, microcephalic, pathology, different species= unsure of where it falls on our phylogenic tree PREHISTORIC ART (note Lascaux cave, France) >600 pictures of animals, mostly horses don’t know purpose possible sympathetic magic cultural symbolism? means of communication ideas? pictograph- painting on surface like a cave wall petroglyph- design carved into rock or other surface HUMAN ORIGINS Associated models: (from book as per your syllabus) multiregional model- evolution happened 1.8 mya in Africa from a single lineage but that changes in modern human anatomy happened by way of gene flow as archaic humans moved across the Old World. -

Phylogenetic Analysis of the Calvaria of Homo Floresiensis Valéry Zeitoun, Véronique Barriel, Harry Widianto
Phylogenetic analysis of the calvaria of Homo floresiensis Valéry Zeitoun, Véronique Barriel, Harry Widianto To cite this version: Valéry Zeitoun, Véronique Barriel, Harry Widianto. Phylogenetic analysis of the calvaria of Homo floresiensis. Comptes Rendus Palevol, Elsevier Masson, 2016, 10.1016/j.crpv.2015.12.002. hal-01290521 HAL Id: hal-01290521 https://hal.sorbonne-universite.fr/hal-01290521 Submitted on 18 Mar 2016 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. Distributed under a Creative Commons Attribution - NonCommercial - NoDerivatives| 4.0 International License G Model PALEVO-924; No. of Pages 14 ARTICLE IN PRESS C. R. Palevol xxx (2016) xxx–xxx Contents lists available at ScienceDirect Comptes Rendus Palevol w ww.sciencedirect.com Human palaeontology and prehistory Phylogenetic analysis of the calvaria of Homo floresiensis Analyse phylogénétique de la calvaria de Homo floresiensis a,∗ b c Valéry Zeitoun , Véronique Barriel , Harry Widianto a UMR 7207 CNRS–MNHN–Université Paris-6, Sorbonne universités, Centre de recherche sur la paléobiodiversité et les e paléoenvironnements, Université Pierre-et-Marie-Curie, T. 46-56, 5 étage, case 104, 4, place Jussieu, 75252 Paris cedex 05, France b UMR 7207 CNRS–MNHN–Université Paris-6, Sorbonne universités, Centre de recherche sur la paléobiodiversité et les paléoenvironnements, 8, rue Buffon, 75252 Paris cedex 05, France c Directorate of Cultural properties and Museums, Komplek Kemdikbud, Gedung E Lt. -

Craniofacial Morphology of Homo Floresiensis: Description, Taxonomic
Journal of Human Evolution 61 (2011) 644e682 Contents lists available at SciVerse ScienceDirect Journal of Human Evolution journal homepage: www.elsevier.com/locate/jhevol Craniofacial morphology of Homo floresiensis: Description, taxonomic affinities, and evolutionary implication Yousuke Kaifu a,b,*, Hisao Baba a, Thomas Sutikna c, Michael J. Morwood d, Daisuke Kubo b, E. Wahyu Saptomo c, Jatmiko c, Rokhus Due Awe c, Tony Djubiantono c a Department of Anthropology, National Museum of Nature and Science, 4-1-1 Amakubo, Tsukuba-shi, Ibaraki Prefecture Japan b Department of Biological Sciences, The University of Tokyo, 3-1-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan c National Research and Development Centre for Archaeology, Jl. Raya Condet Pejaten No 4, Jakarta 12001, Indonesia d Centre for Archaeological Science, School of Earth and Environmental Sciences, University of Wollongong, Wollongong, NSW 2522, Australia article info abstract Article history: This paper describes in detail the external morphology of LB1/1, the nearly complete and only known Received 5 October 2010 cranium of Homo floresiensis. Comparisons were made with a large sample of early groups of the genus Accepted 21 August 2011 Homo to assess primitive, derived, and unique craniofacial traits of LB1 and discuss its evolution. Prin- cipal cranial shape differences between H. floresiensis and Homo sapiens are also explored metrically. Keywords: The LB1 specimen exhibits a marked reductive trend in its facial skeleton, which is comparable to the LB1/1 H. sapiens condition and is probably associated with reduced masticatory stresses. However, LB1 is Homo erectus craniometrically different from H. sapiens showing an extremely small overall cranial size, and the Homo habilis Cranium combination of a primitive low and anteriorly narrow vault shape, a relatively prognathic face, a rounded Face oval foramen that is greatly separated anteriorly from the carotid canal/jugular foramen, and a unique, tall orbital shape. -

The Homo Floresiensis Cranium (LB1): Size, Scaling, and Early Homo Affinities
The Homo floresiensis cranium (LB1): Size, scaling, and early Homo affinities Adam D. Gordon*, Lisa Nevell, and Bernard Wood Department of Anthropology, Center for the Advanced Study of Hominid Paleobiology, The George Washington University, 2110 G Street Northwest, Washington, DC 20052 Edited by David Pilbeam, Harvard University, Cambridge, MA, and approved February 8, 2008 (received for review October 22, 2007) The skeletal remains of a diminutive small-brained hominin found to endocranial size (as it scales with body size) (3, 5, 6, 11) and in Late Pleistocene cave deposits on the island of Flores, Indonesia brain component size (as they scale with endocranial size) (14), were assigned to a new species, Homo floresiensis [Brown P, et al. but, to date, no study has considered the scaling of cranial vault (2004) A new small-bodied hominin from the Late Pleistocene of shape and cranial size when assessing morphological similarity Flores, Indonesia. Nature 431: 1055–1061]. A dramatically different between LB1, modern humans, and fossil hominins. Because the interpretation is that this material belongs not to a novel hominin LB1 cranium is so small relative to modern humans and most taxon but to a population of small-bodied modern humans af- fossil Homo, morphological analyses must take into account how fected, or unaffected, by microcephaly. The debate has primarily cranial shape scales with cranial size because this relationship focused on the size and shape of the endocranial cavity of the type may not be isometric. However, care must be taken when doing specimen, LB1, with less attention being paid to the morphological this, because LB1 falls well outside the size range used to evidence provided by the rest of the LB1 cranium and postcranium, generate regression slopes. -

Homo Floresiensis
Homo floresiensis CHARLES J. VELLA 2016 CALIFORNIA ACADEMY OF SCIENCE DOCENTS GROUP DOWNLOADABLE AT WEBSITE: WWW.CHARLESJVELLAPHD.COM THANKS: L. AIELLO, D. FALK, G. HURLEY Every once in a while, there comes to light a fossil that shakes the foundation of paleoanthropology to its very core and forces us to reconsider what we thought we knew about human evolution. —Donald C. Johanson, Lucy’s Legacy This applies to Homo floresiensis Flores legend of Ebu Gogo There were legends about the existence of little people on the island of Flores, Indonesia. They were called the Ebu Gogo. The islanders describe Ebu Gogo as being about one meter tall, hairy and prone to "murmuring" to each other in some form of language. Discovery 2003 Homo floresiensis, (“the hobbit,”) found in a late Pleistocene context at the cave of Liang Bua by Michael Morwood’s group 2003: Associated with a core and flake assemblage that extended back to ca 95 ka LB1 originally dated to 38 to 13 ka; Lived there from 74 to 17 ka according to original conclusions. An arm bone provisionally assigned to H. floresiensis is about 74,000 years old 2016: new geological assessment places H. floresiensis between 100,000 and 60,000 years old. Measurements of the decay of radioactive elements in an arm bone from the partial skeleton indicate that the find dates to between 86,900 and 71,500 years ago. Until now, researchers suspected these bones were only about 18,000 years old. Later excavations that have dated more rock and sediment around the remains now suggest that hobbits were gone from the cave by 50,000 years ago, according to a study published in Nature on 30 March 2016. -

“Politics” and “Religion” in the Upper Paleolithic: a Voegelinian Analysis of Some Selected Problems
“Politics” and “Religion” in the Upper Paleolithic: A Voegelinian Analysis of Some Selected Problems DRAFT ONLY Barry Cooper University of Calgary Paper prepared for APSA Annual Meeting Seattle WA September, 201 2 Outline 1. Introduction 2. Philosophy of consciousness 3. “Politics” 4. “Religion 5. Conclusions 3 “Politics” and “Religion” in the Upper Paleolithic 1. Introduction The Voegelinian analysis referred to in the title refers primarily to two elements of the political science of Eric Voegelin. The first is his philosophy of consciousness, systematically developed first in Anamnesis.1 The second is his concept of compactness and differentiation of experience and symbolization. It will be necessary to touch upon a few other Voegelinian concepts, notably his understanding of “equivalence,” but for reasons of space only a summary presentation is possible. A second preliminary remark: the terms “Religion” and “Politics” are in quotation marks because their usage in the context of the Upper Paleolithic is anachronistic, though not entirely misleading. The meaning of these terms is commonsensical, not technical, and is meant to indicate what Clifford Geertz once called “oblique family-resemblance connections” among phenomena.2 Third, as a matter of chronology the Upper Paleolithic conventionally refers to the period between 50,000 and 10,000 years ago (50KYBP- 1 Voegelin refined his analysis of consciousness in the last two volumes of Order and History. These changes are ignored on this occasion. 2 Geertz, Life Among the Anthros, ed. Fred Inglis (Princeton: Princeton University Press, 2010), 224. 4 10KYBP). It corresponds in Eurasian periodization approximately to the Later Stone Age in Africa. -

Human Origin Sites and the World Heritage Convention in Eurasia
World Heritage papers41 HEADWORLD HERITAGES 4 Human Origin Sites and the World Heritage Convention in Eurasia VOLUME I In support of UNESCO’s 70th Anniversary Celebrations United Nations [ Cultural Organization Human Origin Sites and the World Heritage Convention in Eurasia Nuria Sanz, Editor General Coordinator of HEADS Programme on Human Evolution HEADS 4 VOLUME I Published in 2015 by the United Nations Educational, Scientific and Cultural Organization, 7, place de Fontenoy, 75352 Paris 07 SP, France and the UNESCO Office in Mexico, Presidente Masaryk 526, Polanco, Miguel Hidalgo, 11550 Ciudad de Mexico, D.F., Mexico. © UNESCO 2015 ISBN 978-92-3-100107-9 This publication is available in Open Access under the Attribution-ShareAlike 3.0 IGO (CC-BY-SA 3.0 IGO) license (http://creativecommons.org/licenses/by-sa/3.0/igo/). By using the content of this publication, the users accept to be bound by the terms of use of the UNESCO Open Access Repository (http://www.unesco.org/open-access/terms-use-ccbysa-en). The designations employed and the presentation of material throughout this publication do not imply the expression of any opinion whatsoever on the part of UNESCO concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. The ideas and opinions expressed in this publication are those of the authors; they are not necessarily those of UNESCO and do not commit the Organization. Cover Photos: Top: Hohle Fels excavation. © Harry Vetter bottom (from left to right): Petroglyphs from Sikachi-Alyan rock art site. -

100,000–11,000 Years Ago 75°
Copyrighted Material GREENLAND ICE SHEET 100,000–11,000 years ago 75° the spread of modern humans Berelekh 13,400–10,600 B ( E around the world during A ALASKA la R I ) SCANDINAVIAN n I e Bluefish Cave d N Arctic Circle G g 16,000 d ICE SHEET b G the ice age N i 25,000–10,000 r r i I d I b g e A R d Ice ) E n -fr SIBERIA a Dry Creek e l e B c All modern humans are descended from populations of ( o 35,000 Dyuktai Cvae 13,500 rri do 18,000 r Homo sapiens that lived in Africa c. 200,000 years ago. op LAURENTIDE en s ICE SHEET 1 Malaya Sya Around 60,000 years ago a small group of humans left 4 CORDILLERAN ,0 Cresswell 34,000 0 Africa and over the next 50,000 years its descendants 0 ICE SHEET – Crags 1 2 14,000 colonized all the world’s other continents except Antarctica, ,0 Wally’s Beach 0 Paviland Cave Mal’ta 0 EUROPE Mezhirich Mladecˇ in the process replacing all other human species. These 13,000–11,000 y 29,000 Denisova Cave 24,000 . 15,000 a 33,000 45,000 . Kostenki 41,000 migrations were aided by low sea levels during glaciations, Willendorf 40,000 Lascaux 41,700–39,500 which created land bridges linking islands and continents: Kennewick Cro Magnon 17,000 9,300 45° humans were able to reach most parts of the world on foot. Spirit 30,000 Cave Meadowcroft Altamira It was in this period of initial colonization of the globe that 10,600 Rockshelter 14,000 16,000 Lagar Velho Hintabayashi Tianyuan JAPAN modern racial characteristics evolved. -

And the Evolution of Homo Floresiensis
1 2 Primate brains, the ‘island rule’ and the evolution of Homo floresiensis 3 Stephen H. Montgomery 4 Dept. of Genetics, Evolution and Environment, University College London, Gower Street, 5 London, WC1E 6BT. 6 Email: [email protected] 7 Tel: +442076792170 8 Key words: Homo floresiensis, brain size, the island rule, dwarfism, primates 9 Running head: Primate brains & the island rule 10 11 12 13 14 15 16 17 18 Summary 19 The taxonomic status of the small bodied hominin, Homo floresiensis, remains controversial. 20 One contentious aspect of the debate concerns the small brain size estimated for specimen LB1 21 (Liang Bua 1). Based on intraspecific mammalian allometric relationships between brain and 22 body size it has been argued that the brain of LB1 is too small for its body mass and is therefore 23 likely to be pathological. The relevance and general applicability of these scaling rules has, 24 however, been challenged, and it is not known whether highly encephalised primates adapt to 25 insular habitats in a consistent manner. Here, an analysis of brain and body evolution in seven 26 extant insular primates reveals that although insular primates follow the ‘island rule’, having 27 consistently reduced body masses compared to their mainland relatives, neither brain mass or 28 relative brain size follow similar patterns, contrary to expectations that energetic constraints will 29 favour decreased relative brain size. Brain:body scaling relationships previously used to assess 30 the plausibility of dwarfism in H. floresiensis tend to underestimate body masses of insular 31 primates. In contrast, under a number of phylogenetic scenarios, the evolution of brain and body 32 mass in H.