Abutilon Theophrasti) Effect on Corn David P
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

PDF-Document
Supplementary Table 1. Botanical sources of kaempferol/glycosides. Species Family Kaempferol/glycosides References kaempferol 3-O-β-glucopyranoside, Abutilon theophrasti Malvaceae [1] kaempferol 7-O-β-diglucoside Acaenasplendens Rosaceae 7-O-acetyl-3-O-β-D-glucosyl-kaempferol [2] kaempferol 3-O-α-L-rhamnopyranosyl- Aceriphyllumrossii Saxifragaceae [3] (1→6)-β-D-glucopyranoside, kaempferol Acacia nilotica Leguminosae Kaempferol [4] kaempferol 3-O-(6-trans-p-coumaroyl)-β- glucopyranosyl-(12)-β-glucopyranoside- 7-O-α-rhamnopyranoside, kaempferol 7- Aconitum spp Ranunculaceae O-(6-trans-p-coumaroyl)-β- [5] glucopyranosyl-(13)-α- rhamnopyranoside-3-O-β- glucopyranoside Kaempferol 3-O-β-(2' '- Aconitum paniculatum Ranunculaceae [6] acetyl)galactopyranoside Kaempferol, kaempferol 3-O-β-D- galactopyranoside, kaempferol 3-O-α-L- Actinidia valvata Actinidiaceae rhamnopyranosyl-(1→3)-(4-O-acetyl-α-L- [7] rhamnopyranosyl)-(1→6)-β-D- galactopyranoside. kaempferol 7-O-(6-trans-p-coumaroyl)-β- glucopyranosyl-(13)-α- Aconitum napellus Ranunculaceae [8] rhamnopyranoside-3-O-β- glucopyranoside Kaempferol 3-O-α-L –rhamnopyranosyl- (1→6)-[(4-O-trans-p-coumaroyl)-α-L - Adina racemosa Rubiaceae [9] rhamnopyranosyl (1→2)]-(4-O-trans-p- coumaroyl)-β-D-galactopyranoside Allium cepa Alliaceae Kaempferol [10] Kaempferol 3-O-[2-O-(trans-3-methoxy- 4-hydroxycinnamoyl)-β-D- Allium porrum Alliaceae [11] galactopyranosyl]-(1→4)-O-β-D- glucopyranoside, kaempferol glycosides Aloe vera Asphodelaceae Kaempferol [12] Althaea rosea Malvaceae Kaempferol [13] Argyreiaspeciosa -

ISTA List of Stabilized Plant Names 7Th Edition
ISTA List of Stabilized Plant Names th 7 Edition ISTA Nomenclature Committee Chair: Dr. M. Schori Published by All rights reserved. No part of this publication may be The Internation Seed Testing Association (ISTA) reproduced, stored in any retrieval system or transmitted Zürichstr. 50, CH-8303 Bassersdorf, Switzerland in any form or by any means, electronic, mechanical, photocopying, recording or otherwise, without prior ©2020 International Seed Testing Association (ISTA) permission in writing from ISTA. ISBN 978-3-906549-77-4 ISTA List of Stabilized Plant Names 1st Edition 1966 ISTA Nomenclature Committee Chair: Prof P. A. Linehan 2nd Edition 1983 ISTA Nomenclature Committee Chair: Dr. H. Pirson 3rd Edition 1988 ISTA Nomenclature Committee Chair: Dr. W. A. Brandenburg 4th Edition 2001 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 5th Edition 2007 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 6th Edition 2013 ISTA Nomenclature Committee Chair: Dr. J. H. Wiersema 7th Edition 2019 ISTA Nomenclature Committee Chair: Dr. M. Schori 2 7th Edition ISTA List of Stabilized Plant Names Content Preface .......................................................................................................................................................... 4 Acknowledgements ....................................................................................................................................... 6 Symbols and Abbreviations .......................................................................................................................... -

US EPA, Pesticide Product Label, FL-AN405F, 07/19/2012
UNITED STATES ENVIRONMENTAL PROTECTION AGENCY WASHINGTON DC 20460 JUL102012 OFFICE OF CHEMICAL SAFETY AND POLLUTION PREVENTION Sara Ghantous, Manager Falcon Lab LLC 4900 Este Ave Cincinnati, OH 45232 Subject FL-AN405F EPA Registration No 79766-4 Label Amendment to add additional producers to CSF, add alternate packaging size to the label and amend the Environmental Hazards statement by adding the point source discharge statement for large containers Decision # 462775 Application Dated March 15, 2012 Dear Mr Ghantous The amendment referred to above, submitted in connection with registration under FIFRA section 3(c)(5), is acceptable provided that you 1 Submit and/or cite all data required for registration/reregistration of your product under FIFRA section 3(c)(5) when the Agency requires all registrants of similar products to submit such data 2 Submit three (3) copies of your final printed labeling before you release the product for shipment Final printed labeling means the label or labeling of the product when distributed or sold Clearly legible reproductions or photo reductions will be accepted for unusual labels, such as those silk-screened directly onto glass or metal containers or large bags or drum labels If these conditions are not complied with, the registration will be subject to cancellation in accordance with FIFRA section 6(b) Your release for shipment of the product bearing the amended labeling constitutes acceptance of these conditions Should you have any questions, you may contact Ms Menyon Adams directly at 703 347 8496 -

First Evidence of the Occurrence of Turnip Mosaic Virus in Ukraine and Molecular Characterization of Its Isolate
Received: 7 September 2017 | Accepted: 1 March 2018 DOI: 10.1111/jph.12703 ORIGINAL ARTICLE First evidence of the occurrence of Turnip mosaic virus in Ukraine and molecular characterization of its isolate Oleksiy Shevchenko1 | Ryosuke Yasaka2,3 | Olha Tymchyshyn1 | Tetiana Shevchenko1 | Kazusato Ohshima2,3 1Virology Department, ESC “Institute of Biology and Medicine”, Taras Shevchenko Abstract National University of Kyiv, Kyiv, Ukraine A total of 54 samples of Brassicaceae crops showing symptoms of mosaic, mottling, 2 Laboratory of Plant Virology, Department vein banding and/or leaf deformation were collected in Kyiv region (northern central of Applied Biological Sciences, Faculty of Agriculture, Saga University, Saga, Japan part of Ukraine) in 2014–2015. A half of collected samples was found to be infected 3The United Graduate School of Agricultural with Turnip mosaic virus (TuMV), and TuMV was detected in samples from Brassica Sciences, Kagoshima University, Kagoshima, oleracea var. capitata (cabbage), Raphanus sativus, Brassica juncea, Raphanus sp., Japan Sinapis alba, Camelina sativa and Bunias orientalis (weed). The full- length sequence of Correspondence the genomic RNA of a Ukrainian isolate (UKR9), which was isolated from cabbage, O. Shevchenko, Virology Department, ESC “Institute of Biology and Medicine”, Taras was determined. Recombination analysis of UKR9 isolate showed that this isolate Shevchenko National University of Kyiv, was an interlineage recombinant of world- Brassica and Asian- Brassica/Raphanus phy- Kyiv, Ukraine. -

Abutilon Theophrasti
Abutilon theophrasti Abutilon theophrasti Velvetleaf, China jute Introduction The genus Abutilon contains approximately 160 species worldwide, primarily in tropical and subtropical areas. In China, ten species and three varieties occur throughout the country. Abutilon species are cultivated for medicinal and ornamental purposes as well as a fiber source[44]. Species of Abutilon in China Abutilon theophrasti buds and flower. (Photo courtesy of LBJWC.) Scientific Name Scientific Name A. crispum (Linn.) Medicus A. paniculatum Hand.-Mazz. A. gebauerianum Hand.-Mazz. A. roseum Hand.-Mazz. A. guineens (Schumacher)† A. sinense Oliv. A. hirtum (Lamk.) Sweet A. striatum Dickson. A. indicum (Linn.) Sweet A. theophrasti Medicus † from the revised Flora of China (FOC)[170]; others from Flora Reipublicae Popularis Sinicae (Flora of People’s Republic of China, FRPS)[44] 5-10 cm long, densely stellate pubescent Taxonomy on both surfaces, with minutely crenate Family: Malvaceae margin, long acuminate apex and Genus: Abutilon Miller Abutilon theophrasti. (LBJWC) cordate base. Petiole is 3-12 cm long with stellate hairs. The stipule is shed Description Petals are yellow, obovate, and about 1 early. Blooming from July to August, Abutilon theophrasti is an annual cm in length. Fruits are semi-globose yellow flowers are produced solitarily in subshrub-like herb that can reach a capsules, about 2 cm in diameter and leaf axils. The pedicel is pubescent, 1-3 height of 1-2 m. The stem and twigs 1.2 cm in length, with 15-20 scabrous cm long, with a knot near the apex. The are covered with fine hairs. The velvety, mericarps bearing two long awns at calyx is cup-shaped, densely puberulous, heart-shaped leaves are alternate, about the apex. -

Abutilon Theophrasti ) Seeds
Weed Science, 53:479±488. 2005 Corn competition alters the germinability of velvetleaf (Abutilon theophrasti ) seeds Robert E. Nurse Velvetleaf is a troublesome annual weed in many cropping systems of the United Department of Crop and Soil Sciences, Cornell States and Canada. Differences in the growing environment of parent plants can University, Ithaca, NY 14853. Present address: in¯uence the number, structure, germinability, and viability of seeds produced. Thus, Agriculture and Agri-food Canada, Greenhouse and the effects across a range of competitive environments and corn planting dates on Crops Processing Centre, Harrow, ON N0R 1G0, velvetleaf seed production, germination, and seed coat weight were examined under Canada ®eld conditions. Seed production of velvetleaf increased with increasing biomass. Total velvetleaf reproductive output was reduced in competition with corn compared Antonio DiTommaso with monoculture stands. Corn planting date had no effect on the dormancy status Corresponding author. Department of Crop and of seeds, but increased competition from corn resulted in up to a 30% decrease in Soil Sciences, Cornell University, Ithaca, NY 14853; the proportion of seeds that were dormant. Seed and seed coat weights also decreased [email protected] for plants of velvetleaf grown in competition with corn compared with those grown in monoculture. These ®ndings suggest that velvetleaf plants growing in relatively noncompetitive environments, such as along ®eld edges or in ®eld areas with poor crop stands, are likely not only to produce a greater number of seeds but also a greater proportion of seeds that are dormant. This alteration in the dormancy status of velvetleaf seeds in the absence or presence of a crop provides unique opportunities for effective long-term management of the soil seedbank in this species, especially for velvetleaf individuals bordering ®elds or growing in fallow areas that might re- quire more stringent control because of increased seed dormancy. -

Abutilon Theophrasti) and Soybean (Glycine Max) Williiam Charles Akey Iowa State University
Iowa State University Capstones, Theses and Retrospective Theses and Dissertations Dissertations 1989 The oler of competition for light in interference between velvetleaf (Abutilon theophrasti) and soybean (Glycine max) Williiam Charles Akey Iowa State University Follow this and additional works at: https://lib.dr.iastate.edu/rtd Part of the Agricultural Science Commons, Agriculture Commons, and the Agronomy and Crop Sciences Commons Recommended Citation Akey, Williiam Charles, "The or le of competition for light in interference between velvetleaf (Abutilon theophrasti) and soybean (Glycine max) " (1989). Retrospective Theses and Dissertations. 8909. https://lib.dr.iastate.edu/rtd/8909 This Dissertation is brought to you for free and open access by the Iowa State University Capstones, Theses and Dissertations at Iowa State University Digital Repository. It has been accepted for inclusion in Retrospective Theses and Dissertations by an authorized administrator of Iowa State University Digital Repository. For more information, please contact [email protected]. INFORMATION TO USERS The most advanced technology has been used to photo graph and reproduce this manuscript from the microfilm master. UMI films the text directly from the original or copy submitted. Thus, some thesis and dissertation copies are in typewriter face, while others may be from any type of computer printer. The quality of this reproduction is dependent upon the quality of the copy submitted. Broken or indistinct print, colored or poor quality illustrations and photographs, print bleedthrough, substandard margins, and improper alignment can adversely affect reproduction. In the unlikely event that the author did not send UMI a complete manuscript and there are missing pages, these will be noted. -

Host Plants of Pink Hibiscus Mealybug Pink Hibiscus Mealybug (PHM)
Appendix A 1 A Host Plants of Pink Hibiscus Mealybug Pink Hibiscus Mealybug (PHM) Introduction Those hosts recorded with damaging populations of PHM are denoted with a number after the scientific name. They may or may not be economic hosts. The number corresponds to the reference in which the host was stated to bear large numbers of the mealybug, and this reference is given after the host list (Stibick, 1997; Chang and Miller, 1996). Any local survey needs to take into account not only the list given here, but also those local plant species which may prove to be hosts. Since PHM demonstrates apparent changes in host preferences by locality, perhaps as a reflection of changes in habitat, environment, and interactions with the local flora/fauna/predator/parasite complex, a local host list should be designed, based on actual local finds, with this list of value only as a guide in the search for preferred and other local hosts. Notes 1. Some hosts are reported to be attacked at their roots (potatoes, peanuts, beans, cotton, some grasses). 2. Symptoms may vary depending on the host (See Biology). 3. When reviewing this list, keep in mind that the literature may have misidentifications of PHM. Hosts by Scientific and Common Names Scientific Name Common Name Reference Abelmoschus esculentus 5 Okra Mani, 1989 Aberia sp. N/A Chang & Miller, 1996 Abutilon theophrasti (=avicennae) Velvetleaf Hall, 1921 Acacia sp. Acacia Williams, 1986 Acacia nilotica (=arabica)2 Babul Hall, 1921 Acacia farnesiana Huisache Hall, 1921 09/2001-01 Pink Hibiscus Mealybug A-1 PPQ Appendix A: Host Plants of Pink Hibiscus Mealybug (PHM) Hosts by Scientific and Common Names Scientific Name Common Name Reference Acalypha sp. -

Transmissibility and Pathological Effects of the Mosaic Disease
F ebruary, 1925 Research Bulletin No. ~:2 Transmissibility and Pathological Effects of the Mosaic Disease By O. H . ELMER AGRICULTURAL EXPERIMENT STATION IOWA STATE COLLEGE OF AGRICULTURE AND MECHANIC ARTS BOTANY AND PLANT PATHOLOGY SECTION AMES, IOWA 'fABLE OF CONTENTS Page Mosaic transmission ....... ...................... 39 Materials and methods ..... .... .. .... .... .. 41 Experimental data .... ..... _ _ .......... _. 45 Inoculations among species of Solanaceae and Leguminosae ......... .... ................... 45 Inoculations among species of Solanaceae and Cu- curbitaceae ................................. 48 Inoculations among species of Leguminosae and Cu- curbitaceae ................................. 51 Inoculations among species of Compositae and Solanaceae ................................. 52 Inoculations among species of Monocotyledoneae and Dicotyledoneae ...... ............ .... ....... 54 Transmissibility of mosaic from eight other species. 57 Mosaic of Apiwrn gmveolens . ....... ...... __ . 57 Mosaic of Rubt~s strigost~s . ... _. .. 59 Mosaic of Asdepias syriaca. .. 61 Mosaic of Martynia louisian4 . ............... 62 Mosaic of N epeta cataria . .. 63 Mosaic of Abt~tilO1~ theophrasti. .. 63 Mosaic of Euphorbia preslii . .. .. .. ......... 64 Mosaic of Aqt~il egia spp .. ... _. .. 66 Mosaic transmission between Nicotiana tabacurn and Nicotiana glutinosa ..... ........ ........ .... 67 The role of insects in mosaic transmission. .. 69 Pathological effect of mosaic ............... _. .. 71 Masking of symptoms.......... -

Corn and Velvetleaf (<I>Abutilon Theophrasti</I> ) Transpiration In
University of Nebraska - Lincoln DigitalCommons@University of Nebraska - Lincoln Agronomy & Horticulture -- Faculty Publications Agronomy and Horticulture Department 2011 Corn and Velvetleaf (Abutilon theophrasti ) Transpiration in Response to Drying Soil Jared Schmidt University of Nebraska-Lincoln Erin E. Blankenship University of Nebraska-Lincoln, [email protected] John L. Lindquist University of Nebraska-Lincoln, [email protected] Follow this and additional works at: https://digitalcommons.unl.edu/agronomyfacpub Part of the Plant Sciences Commons Schmidt, Jared; Blankenship, Erin E.; and Lindquist, John L., "Corn and Velvetleaf (Abutilon theophrasti ) Transpiration in Response to Drying Soil" (2011). Agronomy & Horticulture -- Faculty Publications. 622. https://digitalcommons.unl.edu/agronomyfacpub/622 This Article is brought to you for free and open access by the Agronomy and Horticulture Department at DigitalCommons@University of Nebraska - Lincoln. It has been accepted for inclusion in Agronomy & Horticulture -- Faculty Publications by an authorized administrator of DigitalCommons@University of Nebraska - Lincoln. Weed Science 2011 59:50–54 Corn and Velvetleaf (Abutilon theophrasti ) Transpiration in Response to Drying Soil Jared J. Schmidt, Erin E. Blankenship, and John L. Lindquist* Soil water availability is the most important factor limiting crop yield worldwide. Understanding crop and weed transpiration in response to water supply may provide valuable insight into the mechanisms of crop yield loss in water- limited environments. A greenhouse experiment was conducted to quantify corn and velvetleaf transpiration in response to drying soil. Five plants of each species were well watered by adding back the equivalent water loss each day to reach field capacity, and five plants were subjected to drought stress (dry-down) by not replacing lost water. -

An Annotated Bibliography of Weeds As Reservoirs for Organisms Affecting Crops
RESEARCH BULLETIN 1146 JULY 1982 An Annotated Bibliography of Weeds as Reservoirs for Organisms Affecting Crops Ia. NEMATODES J. S. MANUEL L. E. BENDIXEN R. M. RIEDEL The Ohio State University Ohio Agricultural Research and Development Center Wooster, Ohio CONTENTS * * * * Introduction ------------------------------------------------------------------- 1 Acknowledgments --~------------------------------------------------------------- 2 Bibliography ------------------------------------------------------------------- 3 Index -------------------------------------------------------------------------- 19 All publications of the Ohio Agricultural Research and Development Center are available to all on a nondiscriminatory basis without regard to race, color, national origin, sex, or religious affiliation. The Ohio State University Ohio Agricultural Research and Development Center AGDEX 628-640 7-82-3M AN ANNOTATED BIBLIOGRAPHY OF WEEDS AS RESERVOIRS FOR ORGANISMS AFFECTING CROPS Ia. NEMATODES J. s. Manuel, L. E. Bendixen and R. M. Riedel1 Introduction "An Annotated Bibliography of Weeds as Reservoirs for Organisms Affecting Crops. I. Nematodes" was published in 1979. It contains 184 references published between 1927 and 1976. It represents a modest attempt to bring into one comprehensive volume the available results of research done in this particular area during the period covered. The present'publication is intended as a sequel to the one referred to above, updating ~t through 1980 and including some references missed in the first bibliography. It is hoped that the present bibliography will be found to be a useful reference source, as the first one has been, and that it will facilitate collaboration between nematologists and weed scientists. The format used in the first publica~ion was adopted for this one. The papers are listed alphabetically ~ author, year, title, and source followed by specific data. The index includes an alphabetical listing of authors, weeds, and organisms with notations referring to all relevant entries. -
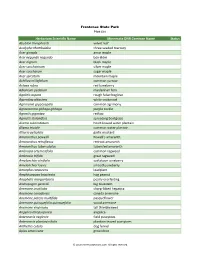
Herbarium Scientific Name Minnesota DNR Common Name Status
Frontenac State Park Plant List Herbarium Scientific Name Minnesota DNR Common Name Status Abutilon theophrasti velvet leaf Acalypha rhomboidea three-seeded mercury Acer ginnala amur maple Acer negundo negundo box elder Acer nigrum black maple Acer saccharinum silver maple Acer saccharum sugar maple Acer spicatum mountain maple Achillea millefolium common yarrow Actaea rubra red baneberry Adiantum pedatum maidenhair fern Agalinis aspera rough false foxglove Ageratina altissima white snakeroot Agrimonia gryposepala common agrimony Agrostemma githago githago purple cockle Agrostis gigantea redtop Agrostis stolonifera spreading bentgrass Alisma subcordatum heart-leaved water plantain Alisma triviale common water plantain Alliaria petiolata garlic mustard Amaranthus powellii Powell's amaranth Amaranthus retroflexus redroot amaranth Amaranthus tuberculatus tubercled amaranth Ambrosia artemisiifolia common ragweed Ambrosia trifida great ragweed Amelanchier alnifolia saskatoon juneberry Amelanchier laevis smooth juneberry Amorpha canescens leadplant Amphicarpaea bracteata hog peanut Anaphalis margaritacea pearly everlasting Andropogon gerardii big bluestem Anemone acutiloba sharp-lobed hepatica Anemone canadensis canada anemone Anemone patens multifida pasqueflower Anemone quinquefolia quinquefolia wood anemone Anemone virginiana tall thimbleweed Angelica atropurpurea angelica Antennaria neglecta field pussytoes Antennaria plantaginifolia plantain-leaved pussytoes Anthemis cotula dog fennel Apios americana groundnut © 2013 MinnesotaSeasons.com.