Jaguar Diet in the Cockscomb Basin Wildlife Sanctuary, Belize1
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

John Ball Zoo Exhibit Animals (Revised 3/15/19)
John Ball Zoo Exhibit Animals (revised 3/15/19) Every effort will be made to update this list on a seasonal basis. List subject to change without notice due to ongoing Zoo improvements or animal care. North American Wetlands: Muted Swans Mallard Duck Wild Turkey (off Exhibit) Egyptian Goose American White pelican (located in flamingo exhibit during winter months) Bald Eagle Wild Way Trail: (seasonal) Red-necked wallaby Prehensile tail porcupine Ring-tailed lemur Howler Monkey Sulphur-crested Cockatoo Red’s Hobby Farm: Domestic goats Domestic sheep Chickens Pied Crow Common Barn Owl Budgerigar (seasonal) Bali Mynah (seasonal) Crested Wood Partridge (seasonal) Nicobar Pigeon (seasonal) John Ball Zoo www.jbzoo.org Frogs: Smokey Jungle frogs Chacoan Horned frog Tiger-legged monkey frog Vietnamese Mossy frog Mission Golden-eyed Tree frog Golden Poison dart frog American bullfrog Multiple species of poison dart frog North America: Golden Eagle North American River Otter Painted turtle Blanding’s turtle Common Map turtle Eastern Box turtle Red-eared slider Snapping turtle Canada Lynx Brown Bear Mountain Lion/Cougar Snow Leopard South America: South American tapir Crested screamer Maned Wolf Chilean Flamingo Fulvous Whistling Duck Chiloe Wigeon Ringed Teal Toco Toucan (opening in late May) White-faced Saki monkey John Ball Zoo www.jbzoo.org Africa: Chimpanzee Lion African ground hornbill Egyptian Geese Eastern Bongo Warthog Cape Porcupine (off exhibit) Von der Decken’s hornbill (off exhibit) Forest Realm: Amur Tigers Red Panda -

The 2008 IUCN Red Listings of the World's Small Carnivores
The 2008 IUCN red listings of the world’s small carnivores Jan SCHIPPER¹*, Michael HOFFMANN¹, J. W. DUCKWORTH² and James CONROY³ Abstract The global conservation status of all the world’s mammals was assessed for the 2008 IUCN Red List. Of the 165 species of small carni- vores recognised during the process, two are Extinct (EX), one is Critically Endangered (CR), ten are Endangered (EN), 22 Vulnerable (VU), ten Near Threatened (NT), 15 Data Deficient (DD) and 105 Least Concern. Thus, 22% of the species for which a category was assigned other than DD were assessed as threatened (i.e. CR, EN or VU), as against 25% for mammals as a whole. Among otters, seven (58%) of the 12 species for which a category was assigned were identified as threatened. This reflects their attachment to rivers and other waterbodies, and heavy trade-driven hunting. The IUCN Red List species accounts are living documents to be updated annually, and further information to refine listings is welcome. Keywords: conservation status, Critically Endangered, Data Deficient, Endangered, Extinct, global threat listing, Least Concern, Near Threatened, Vulnerable Introduction dae (skunks and stink-badgers; 12), Mustelidae (weasels, martens, otters, badgers and allies; 59), Nandiniidae (African Palm-civet The IUCN Red List of Threatened Species is the most authorita- Nandinia binotata; one), Prionodontidae ([Asian] linsangs; two), tive resource currently available on the conservation status of the Procyonidae (raccoons, coatis and allies; 14), and Viverridae (civ- world’s biodiversity. In recent years, the overall number of spe- ets, including oyans [= ‘African linsangs’]; 33). The data reported cies included on the IUCN Red List has grown rapidly, largely as on herein are freely and publicly available via the 2008 IUCN Red a result of ongoing global assessment initiatives that have helped List website (www.iucnredlist.org/mammals). -

Calling All Animal Lovers! Support Your Favorite Animal at America’S Teaching Zoo
CALLING ALL ANIMAL LOVERS! SUPPORT YOUR FAVORITE ANIMAL AT AMERICA’S TEACHING ZOO Don’t miss the chance to play an important role in your Sponsor an animal for yourself, or surprise your friends, family, favorite animal’s life. Our animal sponsorship program allows and co-workers with a gift like no other. Just choose your our community to show their support by making a difference. sponsorship level on the back of this flier and fill out the form. The cost just to feed the animals is more than $100,000 per For questions, call America’s Teaching Zoo at year. By making a donation to sponsor an animal, you’re (805) 378-1441. helping to pay for the care and feeding of furred, feathered, and scaled residents of America’s Teaching Zoo. Animal Ambassadors come in all shapes and sizes America’s Teaching Zoo at Moorpark College houses over 130 animals of more than 90 species. Many of these animals are endangered in the wild, while others have found a permanent home here or otherwise could not survive on their own. This outstanding animal family is utilized in the instruction of students in the Exotic Animal Training and Management Program (EATM). Your sponsorship dollars will go directly to help one of our many animals listed below. BIRDS REPTILES & AMPHIBIANS MAMMALS Bengal Tiger Red Crested Turaco Leopard Gecko Opossum Hamadryas Baboon White Cheeked Turaco Eastern Box Turtle Domestic Rabbit Concolor Gibbon Double Yellow-Headed Bearded Dragon Domestic Dog Pot Bellied Pig Amazon Parrot Kenyan Sand Boa Hedgehog Nigerian Dwarf Goat Blue-fronted -
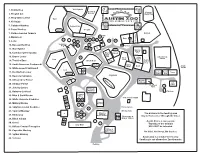
Austin Zoo Map V5 Copy
1. Prairie Dog Wolf Hybrid Serval & Bobcat Visayan 2. Ringtail Cat Warty Pig 3. Ring-tailed Lemur Tiger 4. Kinkajou 5. Colobus Monkey Cougar Cub 6. Patas Monkey Tiger Pool Cougar 7. Golden-handed Tamarin Ostrich Owl 8. Marmoset 23 Emu 19 26 25 23 21 24 28 15 9. Lorie 20 Pig White-tailed Deer Yorkshire 20 27 4 10. Russian Red Fox Pig 23 Bobcat 34 18 12 11. Wolf Hybrid Pool Nigerian Goat & 12. Common Green Iguana Black Bobcat Pig Bear Cub 13. Brown Lemur 31 Pygmy Axis Deer & Boer Goat & Llama Lion Goat Pig Pygmy 14. Tortoise Barn Black Bear Fox Goat & Sheep 15. South American Coatimundi Bear Pool Dairy Barn Goat Train 16. White-nosed Coatimundi Tracks Lion 17 15 17. Red Ruffed Lemur 3 American Prairie Turkey Lion Pool Alligator Dog 18. Red-lored Amazon Capybara Capybara Pool 19. African Grey Parrot Reptile 14 House Train 20. Amazon Parrot Depot Peacock 4 Barn 21. Jenday Conure 4 Primate Pavilion Chicken 22. Rainbow Lorikeet 8 Primate 23. Blue & Gold Macaw Palace 13 Concession Stand 24. White Umbrella Cockatoo 12 32 33 11 Keeper 25. Military Macaw Building 10 9 29 26. Sulphur-crested Cockatoo 7 Picnic Grove 16 The Big Sink 27. Squirrel Monkey 4 Education The animals in the feeding area 7 56 Building/ 28. Binturong Events Pavilion 3 Restrooms & may be fed over or through the fence. Diaper Station 29. Black Caique 30 30 2 Austin Zoo is a rescue zoo. 30. Genet 4 1 Gray Wolf Taunting of the animals 31. African Crested Porcupine WILL NOT be tolerated. -

Grasslands & Boreal Forest
MAP Across Portage Footbridge Assiniboine Park Drive McFeetors INDOOR SPACES Greyed out areas temporarily Closed OUTDOOR ANIMALS Heavy Horse Chimney Centre Swift Tower GRASSLANDS & GRASSLANDS & BOREAL FOREST BOREAL FOREST Bison PRAIRIE OUTPOST: Tundra Leatherdale Red fox Grill International Burrowing owl Polar Bear Wolf ASIA Conservation Centre JOURNEY TO CHURCHILL Lynx Gibbon Pavilion OPEN Elk Special GATEWAY TO THE ARCTIC: RANGE JOURNEY TO Cub Care Events Gift Shop Centre Polar bear, seal, Sea Ice Passage Stone sheep CHURCHILL Entrance Washrooms 360 Aurora Borealis Theatre Serow Closed - LEATHERDALE INTERNATIONAL Bighorn sheep Nursing Station POLAR BEAR CONSERVATION CENTRE AUNT SALLY’S FARM AUNT Food Service TUNDRA GRILL: Dine in and Takeout options SALLY’S available. Daily 9 a.m. – 5 p.m. Miniature horse FARM Picnic Site Goat Gateway Arctic Treasures Gift Shop to the Kinsmen Picnic Tent Closed - Polar Playground, birthday parties Llama Arctic Discovery Centre Equipment Rental JOURNEY TO CHURCHILL OPEN RANGE Snowy owl Carolyn Education Bike Parking Sifton Barn & Program McFeetors HEAVY HORSE CENTRE: Centre Reindeer Doug Harvey, DISCOVERY Corpell’s Hydration Stations Paddock, Barn Arctic fox Jan Shute and ZONE Family Centre SOUTH Parking Muskox AMERICA Toucan ASIA Ridge Seal Parks Canada Winnipeg Transit GIBBON PAVILION: oTENTik Polar bear Electric Car Charging SOUTH AMERICA OPEN RANGE Commissary Road First Aid Heavy horse TOUCAN RIDGE: AED Kinkajou, ocelot, red panda, and more Ibex Llama Sensory Inclusive Zone Zoo Drive AUNT SALLY’S FARM Pronghorn Partial pathway closed. Kangaroo Pathway closed. No visitor access. Demoiselle Crane Prairie Open daily 9 a.m. – 5 p.m. Outpost ASIA Welcome to our smoke free Zoo. -

I METHODS of NICHE PARTITIONING BETWEEN
METHODS OF NICHE PARTITIONING BETWEEN ECUADORIAN CARNIVORES AND HABITAT PREFERENCE OF THE MARGAY ( LEOPARDUS WIEDII ) Anne-Marie C. Hodge A Thesis Submitted to the University of North Carolina Wilmington in Partial Fulfillment for the Requirements for the Degree of Master of Science Department of Biology and Marine Biology University of North Carolina Wilmington 2012 Approved By: Advisory Committee Travis Knowles Steven Emslie Marcel van Tuinen Brian Arbogast Chair Accepted by Dean, Graduate School i TABLE OF CONTENTS ABSTRACT ................................................................................................................................... iv DEDICATION .................................................................................................................................v LIST OF TABLES ......................................................................................................................... vi LIST OF FIGURES ...................................................................................................................... vii CHAPTER 1: MARGAY ACTIVITY PATTERNS AND DENSITY............................................1 Introduction ..........................................................................................................................1 Methods................................................................................................................................5 Study Location .........................................................................................................5 -

Monkey Sanctuary
Monkey Sanctuary This centre is located in an isolated area of the Ecuadorian rainforest. The centre was established in 2006 and works in rescuing, rehabilitating and when possible releasing animals back to their natural environment. The centre works in partnership with the Ecuadorian Environmental Police (UPMA) and the Ministry of Environment in the fight against trafficking, abuse, capture and hunting of wild animals. Animal welfare is not often a high priority for many Ecuadorians and many do not understand the problems associated with keeping wild animals as domestic pets as they often become depressed/aggressive and as a result are neglected and abused and many of the animals arriving at the centre have been mistreated with either physical and/or psychological injuries. The rescue centre covers an area of over 100 hectares on the land of the Alvarez Guatactuca family. This is a Quichua family whose ancestral line over this land and area stems back hundreds of years. As a family-run rescue centre, they have a great understanding of the local community, their culture and the plight of their native wild animals. What started as a small centre for monkeys has expanded to a centre that at the request of the Government now takes in all types of wild animals. This is a great opportunity to work not only with monkeys but with a variety of exotic animals. There are currently over 60 animals at the centre which include: primates (squirrel, titi and capuchin monkeys) mammals (peccaries, coati, tayra, kinkajou, margay), reptiles (tortoises) and birds (turkey, parrots and macaws). -

Procyonid (Procyonidae) Care Manual
PROCYONID (Procyonidae) CARE MANUAL CREATED BY THE AZA Small Carnivore Taxon Advisory Group IN ASSOCIATION WITH THE AZA Animal Welfare Committee Procyonid (Procyonidae) Care Manual Procyonid (Procyonidae) Care Manual Published by the Association of Zoos and Aquariums in association with the AZA Animal Welfare Committee Formal Citation: AZA Small Carnivore TAG 2010. Procyonid (Procyonidae) Care Manual. Association of Zoos and Aquariums, Silver Spring, MD. p.114. Original Completion Date: 13 August 2008, 1st revision June 2009, 2nd revision May 2010 Authors and Significant contributors: Jan Reed-Smith, M.A., Columbus Zoo and Aquarium Celeste (Dusty) Lombardi, Columbus Zoo and Aquarium, AZA Small Carnivore TAG (SCTAG) Chair Mike Maslanka, M.S., Smithsonian‟s National Zoo, AZA Nutrition SAG Barbara Henry, M.S., Cincinnati Zoo and Botanical Garden, AZA Nutrition SAG Chair Miles Roberts, Smithsonian‟s National Zoo Kim Schilling, Animals for Awareness Anneke Moresco, D.V.M., Ph.D., UC Davis, University of California See Appendix L for additional contributors to the Procyonid Care Manual. AZA Staff Editors: Lacey Byrnes, B.S. ACM Intern Candice Dorsey, Ph.D., Director of Animal Conservation Cover Photo Credits: Liz Toth Debbie Thompson Cindy Colling Reviewers: Sue Booth-Binczik, Ph.D., Dallas Zoo Denise Bressler, Logan & Abby‟s Fund Kristofer Helgen, Smithsonian Institution Kim Schilling, Animals for Awareness Mindy Stinner, Conservators‟ Center, Inc. Debbie Thompson, Little Rock Zoo Rhonda Votino Debborah Colbert Ph.D., AZA, Vice President of Animal Conservation Paul Boyle Ph.D., AZA, Senior Vice President of Conservation and Education Disclaimer: This manual presents a compilation of knowledge provided by recognized animal experts based on the current science, practice, and technology of animal management. -

Animal Establishment Application
HENDERSON POLICE DEPARTMENT Animal Care & Control Bureau Animal Establishment Permit (includes Retailers & Dealers) Biannual Application 223 Lead Street ♦ P.O. Box 95050 ♦ Henderson,A NV 89009-5050 ♦ (702)267-4501 HENDERSON POLICE DEPARTMENT New Permit Application: Yes No Date of BreederApplication:__________________ Permit Application If No, Current Animal Control Permit Number:____________________________________ Business Name: _______________________________________________________________ Business Address: _____________________________________________________________ Type of Business: ______________________________________________________________ Business Phone #: ______________________________________________________________ Business E-mail address:________________________________________________________ City of Henderson Business License #: ____________________________________________ Business Owner’s Name: ________________________________________________________ Business Owner’s Primary phone #: _______________ Alternate phone #_______________ Business Owner’s Residence Address: _____________________________________________ ______________________________________________________________________________ Business Owner’s DL #: ______________________________________State:_____________ Business Owner’s Date of Birth: _________________________________________________ Responsible Party if not Business Owner:__________________________________________ Responsible Party DL #: ______________________State:_______ Date of Birth:__________ Type -

For Creative Minds
For Creative Minds The For Creative Minds educational section may be photocopied or printed from our website by the owner of this book for educational, non-commercial uses. Cross-curricular teaching activities, interactive quizzes, and more are available online. Go to www.ArbordalePublishing.com and click on the book’s cover to explore all the links. Domestic Pet, Exotic Animal, or Farm Animal? Pets are tame. They are companion animals and live with people in houses and on farms. Cats and dogs are well-known pets. Sometimes people keep exotic animals as pets. Even if people keep “exotic animals” from a young age, they still have wild instincts. Farm animals are raised to produce food (milk, eggs, or meat) or fiber (wool) for humans. Which animals are pets, exotic animals, or farm animals (livestock)? Farm: cow, goat, llama, pig. llama, goat, cow, Farm: Answers: Domestic Pets: cat, dog. Exotic: arctic fox, binturong, Canadian lynx, kinkajou, tiger, wolf. wolf. tiger, kinkajou, lynx, Canadian binturong, fox, arctic Exotic: dog. cat, Pets: Domestic Answers: Sanctuaries Animal sanctuaries are “forever homes.” They are safe places for animals to live for the rest of their lives. The animals will be cared for and fed but will have enough room to roam so they stay healthy. But how and why do animals get there? Animals can get hurt or orphaned. They might be hit by cars or they can be injured projecting themselves from predators. Wild animals that are unable to be returned to their native environment may live in rescue zoos, sanctuaries, or education centers. -

Read an Excerpt
Welcome to The GROW & READ Early Reader Program! The GROW & READ book program was developed under the supervision of reading specialists to develop kids’ reading skills while emphasizing the delight of storytelling. The series was created to help children enjoy learning to read and is perfect for shared reading and reading aloud. These GROW & READ levels will help you choose the best book for every reader. For more information visit growandread.com. Copyright © 2019 by Fabled Films LLC All rights reserved. No part of this book may be used or reproduced in any manner whatsoever without written permission from the publisher except in the case of brief quotations embodied in critical articles or reviews. For information address Fabled Films LLC, 200 Park Avenue South, New York, NY 10003. The [email protected] Published by Fabled Films LLC, New York ISBN: 978-1-944020-25-5 Kooky Kinkajou Library of Congress Control Number: 2018960143 by First Edition: September 2019 1 3 5 7 9 10 8 6 4 2 Tracey Hecht Cover Designed by Jaime Mendola-Hobbie Jacket & Interior Art by Josie Yee Interior Book Design by Aleks Gulan Typeset in Stemple Garamond, Mrs. Ant and Pacific Northwest Illustrations by Printed by Everbest in China Josie Yee fabledfilms.com For information on bulk purchases for promotional use, please contact Consortium Book Sales & Distribution Sales department at [email protected] or 1-866-400-5351. Fabled Films Press New York Chapter 1 “I’m bored!” Bismark said. “There’s nothing to do!” The sugar glider harrumphed and plopped down on a stump. -

Curriculum Vital
Updated 12.6.2014 Kim Yeoman (919) 744 - 9889 [email protected] EDUCATION: The University of North Carolina at Greensboro, NC 2011 - 2014. Master of Science in Biology. North Carolina State University, Raleigh, NC 2003 - 2008. Bachelor of Science in Zoology, cum laude. Minors: Entomology, Environmental Toxicology, Plant Biology. Cape Fear Community College, Wilmington, NC 2000 - 2002. College transfer, automotive and architecture electives. RESEARCH: The University of North Carolina at Greensboro, NC 2011 - 2014 Master's thesis research encompassing field and laboratory experimental design, implementation, troubleshooting, mosquito and dragonfly colony maintenance. Team leader, supervision of workflow, scheduling, and task delegation. Athenix Corporation, Research Triangle Park, NC 2009 - 2010. Biologist. Maintenance of agricultural insect pest colonies, nematodes, bioassays, optimizing insect egg hatch rates, diet preparation, agricultural plant propagation, fertilization and augmentation, greenhouse maintenance, and contamination prevention. Environmental Protection Agency, Research Triangle Park, NC 2008 - 2009. Independent Contractor. Integrated Systems Toxicology Division. RNA extraction for microarray applications, DNA purification and sequencing, directional cloning, high throughput assays, robotics automated liquid handling, bacterial transformation, plasmid induction, mammalian cell culture, transfection and transduction, handling of volatile, biologically hazardous and toxic compounds, equipment calibration, and data compilation. Endangered Species Branch, Fort Bragg, NC 2005 - 2007. Field Technician. Seasonal monitoring, mist-netting, biological sampling, and recording echolocation calls using Pettersson detector and SonoBat software to determine local species populations of bats. TEACHING: Graduate Teaching Assistant, University of North Carolina at Greensboro, NC 2011 - 2014. Graduate Teaching Assistant. BIO 105L Major Concepts of Biology Laboratory, BIO112 Principles of Biology II, BIO 301 Principles of Ecology, BIO302 Principles of Ecology Lab.