I METHODS of NICHE PARTITIONING BETWEEN
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Current Issue
Current Issue Cat Project of the Month - September 2005 The IUCN/SSC Cat Specialist Group's website (www.catsg.org) presents each month a different cat conservation project. Members of the Cat Specialist Group are encouraged to submit a short description of interesting projects For application use this standardised form (an editable word document) Pampas cat ecology and conservation in the Brazilian grasslands The main goal of the study on pampas cats in the Brazilian grasslands is to rise information on the ecology of the species, including data on diseases, genetics and threats. Leandro Silveira, Anah T. A. Jácomo and Mariana Malzoni Furtado Leandro Silveira (photo) is a wildlife biologist (PhD). He is the project leader of the Carnovire Community Project at Emas Naitonal Park and is the president of the Jaguar Conservation Fund. Leandro has been a member of the Cat SG since 2002. Since 2003, he is conduction a study on the papas cat (Photo Jaugar Conservation Fund) [email protected] submitted: 23/09/2005 The pampas cat (Oncifelis colocolo) is widely distributed in a variety of South American habitats. It ranges from the forested Andean slopes of Ecuador, Peru and Bolívia, cloud forest in central Chile, Paraguayan chaco, open woodlands of central, western, northeastern and southern Brazil, the Argentine pampas, southern Patagonia, and the pampas of Uruguay (Cabrera 1957, Vieira, 1955; Ximenez, 1961). In Brazil it is restricted to open habitats and it is found in the Cerrado of central Brazil, the Pantanal of western Mato Grosso do Sul, and in southern open grassland and mangrove habitats. -

Oncilla 1 Oncilla
Oncilla 1 Oncilla Tiger Cat redirects here. For the Tom and Jerry Tales episode, see Tiger Cat (Tom and Jerry Tales). Oncilla[1] Conservation status [2] Vulnerable (IUCN 3.1) Scientific classification Kingdom: Animalia Phylum: Chordata Class: Mammalia Order: Carnivora Family: Felidae Genus: Leopardus Species: L. tigrinus Binomial name Leopardus tigrinus (Schreber, 1775) Oncilla 2 Oncilla range Synonyms Oncifelis tigrinus Felis tigrina The Oncilla (Leopardus tigrinus), also known as the Little Spotted Cat, Tigrillo, Cunaguaro or Tiger Cat, is a small spotted felid found in the tropical rainforests of Central and South America. It is a close relative of the Ocelot and the Margay, and has a rich ochre coat, spotted with black rosettes. The Oncilla is a nocturnal animal that hunts rodents and birds.[3] Appearance The Oncilla resembles the Margay and the Ocelot,[4] but is smaller, with a slender build and narrower muzzle. It grows to 38 to 59 centimetres (15 to 23 in) long, plus a 20 to 42 centimetres (7.9 to 17 in) tail.[5] While this is somewhat longer than the average domestic cat, Leopardus tigrinus is generally lighter, weighing 1.5 to 3 kilograms (3.3 to 6.6 lb).[6] The fur is thick and soft, ranging from light brown to dark ochre, with numerous dark rosettes across the back and flanks. The underside is pale with dark spots and the tail is ringed. The backs of the ears are black with bold ocelli.[4] The rosettes are black or brown, open in the center, and irregularly shaped.[7] The legs have medium-sized spots tapering to smaller spots near the paws.[7] This coloration helps the oncilla blend in with the mottled sunlight of the tropical forest understory. -

Pantanal, Brazil 12Th July to 20Th July 2015
Pantanal, Brazil 12th July to 20th July 2015 Steve Firth Catherine Griffiths This trip was an attempt to see some mammal species that had eluded us on many previous visits to South America. Cats were the main focus, specifically Jaguar and Ocelot, and we were hoping for Giant Anteater as a bonus. When we started planning the trip some ten months in advance, the exchange rate was £1 = R$3.8. The pound strengthened considerably in the intervening period and was £1 = R$5.0 during the visit. This helped to appreciably reduce costs . We flew from London to Campo Grande via Sao Paulo with TAM. There was a 10 hour stopover, but the flight was a great deal cheaper than any offered by other Airlines. On the return leg we flew from Cuiaba to London again via Sao Paulo, again with a long layover. The total Cost per person was £943.35. TAM proved to be more efficient than we had expected (we had had a few memorable difficulties with VARIG 15 years previously) and can be recommended. The Campo Grande to Cuiaba leg was flown with AZUL, booked via their website. The rate quoted, R$546.50 (£70.84 each at the time of booking) for two people, was actually charged to our credit card as US Dollars $546.50. This was noticed immediately and after a call to AZUL in Brazil, they swiftly refunded the first charge and debited the correct amount. AZUL are a low cost carrier, but this was not reflected in their service or punctuality. -
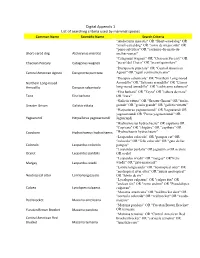
Digital Appendix 1 List of Searching Criteria Used by Mammal Species
Digital Appendix 1 List of searching criteria used by mammal species Common Name Scientific Name Search Criteria "Atelocynus microtis" OR "Short-eared dog" OR "small-eared dog" OR "zorro de oreja corta" OR "perro selvático" OR "cachorro-do-mato-de- Short-eared dog Atelocynus microtis orelhas-curtas" "Catagonus wagneri" OR "Chacoan Peccary" OR Chacoan Peccary Catagonus wagneri "pecarí del Chaco" OR "pecarí quimilero" “Dasyprocta punctate” OR "Central American Central American Agouti Dasyprocta punctata Agouti" OR "agutí centroamericano" “Dasypus sabanicola” OR "Northern Long-nosed Northern Long-nosed Armadillo" OR "Savanna armadillo" OR "Llanos Armadillo Dasypus sabanicola long-nosed armadillo" OR "cachicamo sabanero" “Eira barbara” OR "Tayra" OR "cabeza de mate" Taira Eira barbara OR "irara" “Galictis vittata” OR "Greater Grison" OR "furão- Greater Grison Galictis vittata grande" OR "grisón grande" OR "galictis vittatta" “Herpailurus yagouaroundi” OR Yaguarundi OR yagouaroundi OR "Puma yagouaroundi" OR Yaguarundi Herpailurus yagouaroundi Jaguarundi "Hydrochoerus hydrochaeris" OR capybara OR "Capivara" OR "chigüire" OR "capibara" OR Capybara Hydrochoerus hydrochaeris "Hydrochaeris hydrochaeris" “Leopardus colocolo” OR "pampas cat" OR "colocolo" OR "felis colocolo" OR "gato de las Colocolo Leopardus colocolo pampas" "Leopardus pardalis" OR jaguatirica OR ocelote Ocelot Leopardus pardalis OR ocelot “Leopardus wiedii” OR "margay" OR"Felis Margay Leopardus wiedii wiedii" OR "gato-maracajá" “Lontra longicaudis” OR "neotropical otter" OR "neotropical -

John Ball Zoo Exhibit Animals (Revised 3/15/19)
John Ball Zoo Exhibit Animals (revised 3/15/19) Every effort will be made to update this list on a seasonal basis. List subject to change without notice due to ongoing Zoo improvements or animal care. North American Wetlands: Muted Swans Mallard Duck Wild Turkey (off Exhibit) Egyptian Goose American White pelican (located in flamingo exhibit during winter months) Bald Eagle Wild Way Trail: (seasonal) Red-necked wallaby Prehensile tail porcupine Ring-tailed lemur Howler Monkey Sulphur-crested Cockatoo Red’s Hobby Farm: Domestic goats Domestic sheep Chickens Pied Crow Common Barn Owl Budgerigar (seasonal) Bali Mynah (seasonal) Crested Wood Partridge (seasonal) Nicobar Pigeon (seasonal) John Ball Zoo www.jbzoo.org Frogs: Smokey Jungle frogs Chacoan Horned frog Tiger-legged monkey frog Vietnamese Mossy frog Mission Golden-eyed Tree frog Golden Poison dart frog American bullfrog Multiple species of poison dart frog North America: Golden Eagle North American River Otter Painted turtle Blanding’s turtle Common Map turtle Eastern Box turtle Red-eared slider Snapping turtle Canada Lynx Brown Bear Mountain Lion/Cougar Snow Leopard South America: South American tapir Crested screamer Maned Wolf Chilean Flamingo Fulvous Whistling Duck Chiloe Wigeon Ringed Teal Toco Toucan (opening in late May) White-faced Saki monkey John Ball Zoo www.jbzoo.org Africa: Chimpanzee Lion African ground hornbill Egyptian Geese Eastern Bongo Warthog Cape Porcupine (off exhibit) Von der Decken’s hornbill (off exhibit) Forest Realm: Amur Tigers Red Panda -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Abundance Changes and Activity Flexibility of the Oncilla, Leopardus Tigrinus (Carnivora: Felidae), Appear to Reflect Avoidance of Conflict
ZOOLOGIA 29 (2): 115–120, April, 2012 doi: 10.1590/S1984-46702012000200003 Abundance changes and activity flexibility of the oncilla, Leopardus tigrinus (Carnivora: Felidae), appear to reflect avoidance of conflict Luiz Gustavo R. Oliveira-Santos1, 6, Maurício E. Graipel2, Marcos A. Tortato3, Carlos A. Zucco1, Nilton C. Cáceres4 & Fernando V. B. Goulart5 1 Laboratório de Ecologia e Conservação de Populações, Departamento de Ecologia, Universidade Federal do Rio de Janeiro. Caixa Postal 68020, 21941-590 Rio de Janeiro, RJ, Brazil. 2 Departamento de Ecologia e Zoologia, Universidade Federal de Santa Catarina. 88040-970 Florianópolis, SC, Brazil. 3 Programa de Pós-graduação em Ecologia e Conservação, Setor de Ciências Biológicas, Universidade Federal do Paraná. 81531-980, Curitiba, PR, Brazil. 4 Departamento de Biologia, Universidade Federal de Santa Maria. 97105-900 Santa Maria, RS, Brazil. 5 Programa de Pós-graduação em Ecologia e Conservação, Universidade Federal do Mato Grosso do Sul. 79070-900 Campo Grande, MS, Brazil. 6 Corresponding Author. Email: [email protected] ABSTRACT. We investigated the density and activity of the oncilla, Leopardus tigrinus (Schreber, 1775), a threatened small cat, in the Brazilian Atlantic Forest, using camera-trap data. We described differences in the activity of individuals occurring alone or in sympatry with larger cats. Oncilla presented low densities (7-13 ind./100 km²) and high flexibility in its activity. The oncillas were primarily nocturnal in the absence of other larger cat species – margay, ocelot and puma – but became more diurnal, with a cathemeral activity pattern, when the other cats were present. Oncilla is likely to be in a subordinate position in interactions with larger cats and changes its activity to decrease the chances for interspecific encounters. -

Galictis Cuja Molina, 1782) As Host of Dioctophyme Renale Goeze, 1782 Furão Pequeno (Galictis Cuja Molina, 1782) Como Hospedeiro De Dioctophyme Renale Goeze, 1782
ANIMAL PARASITOLOGY / SCIENTIFIC COMMUNICATION DOI: 10.1590/1808-1657000312016 Lesser Grison (Galictis cuja Molina, 1782) as host of Dioctophyme renale Goeze, 1782 Furão Pequeno (Galictis cuja Molina, 1782) como hospedeiro de Dioctophyme renale Goeze, 1782 Daniela Pedrassani1*, Mayana Worm1, Jéssica Drechmer1, Margareth Cristina Iazzetti Santos1 ABSTRACT: The Dioctophyme renale is a helminth parasite RESUMO: O Dioctophyme renale é um helminto parasita renal of the kidney usually seen in domestic and wild carnivores and observado normalmente em carnívoros domésticos e silvestres e rarely in human beings. This is a report about the parasitism excepcionalmente em seres humanos. Relata-se o parasitismo por D. of D. renale found in the kidney of two roadkill lesser grisons renale em rim de dois furões pequenos (Galictis cuja) encontrados (Galictis cuja) in the North of the state of Santa Catarina, mortos por atropelamento no Norte do estado de Santa Catarina, Brazil. The report of this parasitism in this species is important Brasil. Relatar esse parasitismo nessa espécie é importante, para to complement the records about this native carnivore as a que se possam somar dados relativos a participação deste carnívoro contributor in the epidemiologic chain while host/disseminator nativo na cadeia epidemiológica como hospedeiro/ veiculador desse of this helminth with zoonotic potential. helminto com potencial zoonótico. KEYWORDS: Dioctophyma; wild animal; mustelids; roadkill; PALAVRAS-CHAVE: Dioctophyma; animal silvestre; mustelí- kidney parasitism. deo; atropelamento em rodovia; parasitismo renal. 1Universidade do Contestado (UnC) – Canoinhas (SC), Brazil. *Corresponding author: [email protected] Received on: 04/22/2016. Accepted on: 09/12/2017 Arq. Inst. Biol., v.84, 1-4, e0312016, 2017 1 D. -

The Leopardus Tigrinus Is One of the Smallest Wild Cats in South America; and the Smallest Cat in Brazil (Oliveira-Santos Et Al
Mckenzie Brocker Conservation Biology David Stokes 20 February 2014 Leopardus Tigrinus Description: The Leopardus tigrinus is one of the smallest wild cats in South America; and the smallest cat in Brazil (Oliveira-Santos et al. 2012). L. tigrinus is roughly the size of a domestic house cat, with its weight ranging from 1.8-3.4 kg (Silva-Pereira 2010). The average body length is 710 millimeters and the cat’s tail is roughly one-third of its body length averaging 250 millimeters in length. Males tend to be slightly larger than the females (Gardner 1971). The species’ coat is of a yellowish-brown or ochre coloration patterned prominently with open rosettes (Trigo et al. 2013). Cases of melanism, or dark pigmentation, have been reported but are not as common (Oliveira-Santos et al 2012). These characteristics spots are what give the L. tigrinus its common names of little spotted cat, little tiger cat, tigrina, tigrillo, and oncilla. The names tigrillo, little tiger cat, and little spotted cat are sometimes used interchangeably with other small Neotropical cats species which can lead to confusion. The species is closely related to other feline species with overlapping habitat areas and similar colorations; namely, the ocelot, Leopardus pardalis, the margay, Leopardus weidii, Geoffroys cat, Leopardus geoffroyi, and the pampas cat, Leopardus colocolo (Trigo et al. 2013). Distribution: The L. tigrinus is reported to have a wide distribution from as far north as Costa Rica to as far south as Northern Argentina. However, its exact distribution is still under study, as there have been few reports of occurrences in Central America. -

Leptailurus Serval)
animals Article The Effect of Behind-The-Scenes Encounters and Interactive Presentations on the Welfare of Captive Servals (Leptailurus serval) Lydia K. Acaralp-Rehnberg 1,*, Grahame J. Coleman 1, Michael J. L. Magrath 2, Vicky Melfi 3, Kerry V. Fanson 4 and Ian M. Bland 1 1 Animal Welfare Science Centre, Faculty of Veterinary and Agricultural Sciences, University of Melbourne, Parkville, Victoria 3052, Australia; [email protected] (G.J.C.); [email protected] (I.M.B.) 2 Department of Wildlife Conservation and Science, Zoos Victoria, Parkville, Victoria 3052, Australia; [email protected] 3 Hartpury University, Gloucester GL193BE, UK; vicky.melfi@hartpury.ac.uk 4 Centre for Integrative Ecology, Deakin University, Geelong, Victoria 3216, Australia; [email protected] * Correspondence: [email protected]; Tel.: +61-404-761-714 Received: 13 April 2020; Accepted: 15 April 2020; Published: 24 April 2020 Simple Summary: Live animal encounter programs are an increasingly popular occurrence in the modern zoo. The effects of such encounters on program animal welfare have not been studied extensively to date. The aim of this study was, therefore, to explore animal welfare effects associated with encounter programs in a small felid, the serval, which is commonly involved as a program animal in zoos. Specifically, this study investigated how serval behaviour and adrenocortical activity (level of faecal cortisol metabolites) were affected by short-term variations in encounter frequency. Over the course of the study, the frequency of encounters was manipulated so that servals alternated between four different treatments, involving interactive presentations, behind-the-scenes encounters, both activities combined, or no interaction at all. -

Redalyc.FIRST RECORD of PANTANAL CAT, Leopardus
Mastozoología Neotropical ISSN: 0327-9383 [email protected] Sociedad Argentina para el Estudio de los Mamíferos Argentina Díaz Luque, José A.; Beraud, Valerie; Torres, Pablo J.; Kacoliris, Federico P.; Daniele, Gonzalo; Wallace, Robert B.; Berkunsky, Igor FIRST RECORD OF PANTANAL CAT, Leopardus colocolo braccatus, IN BOLIVIA Mastozoología Neotropical, vol. 19, núm. 2, julio-diciembre, 2012, pp. 299-301 Sociedad Argentina para el Estudio de los Mamíferos Tucumán, Argentina Available in: http://www.redalyc.org/articulo.oa?id=45725085020 How to cite Complete issue Scientific Information System More information about this article Network of Scientific Journals from Latin America, the Caribbean, Spain and Portugal Journal's homepage in redalyc.org Non-profit academic project, developed under the open access initiative Mastozoología Neotropical, 19(2):299-301, Mendoza, 2012 ISSN 0327-9383 ©SAREM, 2012 Versión on-line ISSN 1666-0536 http://www.sarem.org.ar FIRST RECORD OF PANTANAL CAT, Leopardus colocolo braccatus, IN BOLIVIA José A. Díaz Luque1,2, Valerie Beraud2, Pablo J. Torres3, Federico P. Kacoliris2,4,5, Gonzalo Daniele2, Robert B. Wallace6, and Igor Berkunsky2,5,7 1 Urbanización el Coto, Calle Ruiseñor Nº6, 29651 Mijas Costa, Málaga, España [Correspondence: <[email protected]>]. 2 Proyecto de conservación de la Paraba Barba Azul, World Parrot Trust, Casilla 101, Trinidad, Beni, Bolivia. 3 Universidad Nacional de la Patagonia San Juan Bosco, Boulevard Brown 3700, Puerto Madryn, Chubut, Argentina. 4 División Vertebrados, Facultad de Ciencias Naturales y Museo, Universidad Nacional de La Plata, Paseo del Bosque s/n, B1900FWA La Plata, Argentina. 5 CONICET (Consejo Nacional de Investigaciones Científicas y Técnicas), Argentina. -

Jaguar Diet in the Cockscomb Basin Wildlife Sanctuary, Belize1
BIOTROPICA 38(5): 687–690 2006 10.1111/j.1744-7429.2006.00190.x Cockscomb Revisited: Jaguar Diet in the Cockscomb Basin Wildlife Sanctuary, Belize1 Mark Weckel2 Fordham University, Bronx, New York 10458, U.S.A. William Giuliano University of Florida, Gainesville, Florida 32611, U.S.A. and Scott Silver Wildlife Conservation Society, Bronx, New York 10460, U.S.A. ABSTRACT The Cockscomb Basin Wildlife Sanctuary, Belize was established in 1986 following Rabinowitz and Nottingham’s 1986 study on jaguar diet and distribution. In 2002, we reexamined jaguar diet following two decades of mitigated human hunting of jaguar prey. We observed high overlap between historical and current jaguar diet constructs. Nine-banded armadillo remained the most frequently consumed prey item. However, collared peccary consumption increased by nearly20percent while dietary breadth decreased by 60 percent presumably reflecting increased prey availability following reduced human hunting pressure. Key words: Belize; Dasypus novemcinctus;diet;Panthera onca; Tayassu tajacu; tropical moist forest. RABINOWITZ AND NOTTINGHAM’S (1986) INVESTIGATION OF JAGUAR the Belize Audubon Society (BAS) failed to document evidence DIET in the then unprotected Cockscomb Basin, Belize, Central of humans hunting jaguar prey in Guam Bank; however, subsis- America, characterized the local population as opportunistic preda- tence hunters inhabiting villages on the CBWS periphery estab- tors, consuming primary prey items, armadillo (Dasypus novem- lished hunting routes and encampments to the south and west of cinctus), and paca (Agouti paca), relative to availability. The study the Guam Bank study area (Weckel 2005). incited the Belize government to create what is now the Cockscomb We constructed jaguar diet from scats collected from 1 June Basin Wildlife Sanctuary (CBWS) effectively prohibiting hunting to 24 November 2002.