This Article Appeared in a Journal Published by Elsevier. the Attached
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

The DDCPD-2012 Workshop Will Be Held in Conjunction with the 9Th International Conference on Ubiquitous Intelligence and Computing (UIC 2012)
The DDCPD-2012 Workshop will be held in conjunction with The 9th International Conference on Ubiquitous Intelligence and Computing (UIC 2012). Selected papers of the workshop will be published by IEEE-CS Conference Publishing Services (IEEE-DL and EI indexed). Fukuoka, Japan, September 04-07, 2012 Important dates • Paper Submission Deadline: May 31, 2012 (Final) • Authors Notification: June 15, 2012 • Final Manuscript Deadline: June 28, 2012 DDCPD-2012 CPF (text, PDF) Objectives and Scope Many types of device, both generic (computers, cell phones, etc.) and specific (sensors, devices with embedded systems) are able to collect different kinds of data for several different purposes. Often, when designing a system to deal with distributed data, there is the need to explore efficient, fast, precise and/or resilient ways to collect, process and analyse the data. Although most solutions are specific and ad-hoc, tools, techniques, and best practices from one deployment could be used in others, even for other application domains. The purpose of this workshop is to serve as a forum for discussion of issues related to distributed data collection, processing, analysis and dissemination, with emphasis on the following topics (non-exclusive list): • Architectures and systems for distributed data collection, processing, dissemination. • Sensor networks for data collection and processing. • Hardware-based approaches for large scale and sensors' data processing. • Distributed data collection and processing with embedded systems. • Emerging information systems for data collection and processing. • Distributed intelligence. • Extracting knowledge from distributed information systems. • Integration of heterogeneous data sources. • Data collection, processing and dissemination on the cloud, on grid and cluster computers. -
![No.204 (English) [Pdf]](https://docslib.b-cdn.net/cover/3278/no-204-english-pdf-1013278.webp)
No.204 (English) [Pdf]
ISSN 2188-109X 一般社団法人 大 学 英 語 教 育 学 会 ―――――――――――――――――――――――――――――――――――――― March 2019 The Japan Association of College English Teachers No.204 ―――――――――――――――――――――――――――――――――――――― Contents Foreword (Naoko Ozeki) 1 Report from the Committee of 4 Report from Headquarters 2 Academic Affairs Reports from Chapters 5 Foreword Naoko Ozeki Vice-President of JACET Director, Committee of Academic Publication (Meiji University) To JACET members and supporters, Recently, JACET has been going through changes in terms of its evaluation of papers JACET publishes influential articles in the submitted to the JACET Journal and JACET JACET Journal, JACET International International Convention Selected Papers. One of Convention Selected Papers, and JAAL in JACET the most noticeable changes is the area of Proceedings, each of which is published annually, globalization. For example, since we have and provides an incentive for both researchers and established affiliations with various organizations instructors to do further research and improve such as JALT (Japan), AILA (international), their practice. ALAK (Korea), ETA-ROCK (Taiwan), and JACET 通信―――――――――――――――<1>―――――――――――――――――3189 RELC (Singapore), we have invited international of APA format will be a requirement for having a plenary speakers and guest speakers to our paper accepted in JACET publications. We are international conferences and summer seminars. looking forward to reading your future We have also invited these speakers to write submissions to our journals. articles about the topics they talked about at the conferences for the JACET Journals and Selected Papers. We hope that those who could not Report from the JACET Headquarters participate in the conferences or summer seminars will be able to share the main ideas and insights of Secretary General these speakers’ presentations by reading their Yukinari Shimoyama articles. -

National Institute of Technology, Kurume College
6 Campus Map 7 Access Map From Fukuoka Airport Route A: By train + bus 1. Fukuoka Airport to JR Kurume Sta. (60 min by subway and train) 2. JR Kurume Sta. to Kurume College (12 min by bus) Route B: By highway bus + bus 1. Fukuoka Airport to Nishitetsu Kurume Sta. (45 min by highway bus) 2. Nishitetsu Kurume Sta. to Kurume College (20 min by bus) National Institute of Technology, Kurume College From Fukuoka ● Kitakyushu Route A To Tosu To Fukuoka Fukuoka Airport Kurume Saga● ● Oita 3 National Institute of Technology, Kurume College Nagasaki● ●Kumamoto From Fukuoka Route B Nishitetsu Tenjin Omuta Line JR Kagoshima Line Kagoshima JR Kyushu Shinkansen Kyushu Kurume University, School of Medicine ①管理棟 Administration Building ⑯ 熱・材 力 実 験 棟 Thermal Engineering and Chikugo River Strength of Materials Building ②一般共通棟 General Education Building Lodging House for Extracurricular Activities ③一般教室棟 Classroom General Education Building ⑰練心館 Male Dormitory 3 ④ 一 般 文 科・理 科 棟 Laboratory and Office General Education Faculty Building ⑱ 学 生 寄 宿 舎( 筑 水 寮 ) ⑲ 学 生 寄 宿 舎( つ つ じ 寮 ) Female Dormitory Kurume City Hall ⑤ 機 械・材 料 シ ステム 工 学 科 棟 Dept. of Mechanical Engineering and Materials System 210 Engineering Building ⑳ウェーブホール Cafeteria / Store (Wave Hall) ⑥ 電 気 電 子・制 御 情 報 工 学 科 棟 Dept. of Electrical and Electronic Engineering ㉑学生部室 Extracurricular Activities House JR Kurume Sta. and Control and Information Systems Engineering Building ㉒武道場 Martial Arts Gymnasium Research Center for Technology ⑦総合試作技術教育センター ㉓第一体育館 Gymnasium Ⅰ Kurume Interchange ⑧ も の づくり 教 育 センター Manufacturing Education Center ㉔第二体育館 Gymnasium Ⅱ 322 Dept. -

Research Profiles
Kyushu University Program for Leading Graduate Schools Advanced Graduate Program in Global Strategy for Green Asia Research Profiles Bidyut Baran Saha 4. I.I. El-Sharkawy, K. Uddin, T. Miyazaki, B.B. Saha, S. Koyama, J. Miyawaki and S.H. Yoon Adsorption Professor, Green Asia Education Center of ethanol onto parent and surface treated activated Specialization: carbon powders, International Journal of Heat and Thermal Engineering, Heat Transfer Mass Transfer, Vol. 73, pp. 445–455, 2014. Engneering, Engineering of Refrigerating and Air-conditioning 5. S. Jribi, B.B. Saha, S. Koyama and H. Bentaher, Research: Modeling and simulation of an activated carbon–CO2 Thermally powered adsorption four bed based adsorption cooling system, Energy cooling and desalination systems; Energy effi ciency assessment Conversion and Management, Vol. 78, pp. 985-991, E-mail: 2014. [email protected]. 6. K. Habib, B. Choudhury, P.K. Chatterjee and B.B. ac.jp Saha, Study on a solar heat driven dual-mode adsorption chiller, Energy, Vol. 63, pp. 133-141, 2013. Prof. Bidyut Baran Saha obtained his B.Sc. (Hons.) and 7. K. Uddin, T. Miyazaki, S. Koyama and B.B. Saha, M.Sc. degrees from Dhaka University of Bangladesh in 1987 Performance investigation of adsorption–compression and 1990, respectively. He received his Ph.D. in 1997 from hybrid refrigeration systems, International Journal of the Tokyo University of Agriculture and Technology, Japan. Air-Conditioning and Refrigeration, Vol. 21, No. 4, After graduation he worked as an Associate Professor at the 1350024-1 to 1350024-12, 2013. Interdisciplinary Graduate School of Engineering Sciences 8. -
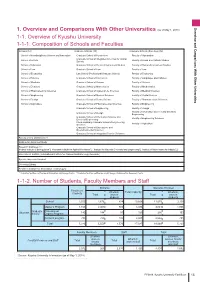
(As of Ay 1, 2018) 1-1. Overview of Kyushu University 1-1-1
Overview and Comparisons With Other Universities 1 vervie and Comarisons ith ther Universities (as of ay 1, 2018) 1-1. Overview of Kyushu University 1-1-1. Composition of Schools and Faculties Schools (12) raduate Schools (18) raduate Schools (Faculties) (16) School of Interdisciplinary Science and Innovation raduate School of Humanities Faculty of Humanities raduate School of Integrated Sciences for lobal School of etters Faculty of Social and Cultural Studies Society School of ducation raduate School of Human-nvironment Studies Faculty of Human-nvironment Studies School of aw raduate School of aw Faculty of aw School of conomics aw School (Professional raduate School) Faculty of conomics School of Science raduate School of conomics Faculty of anguages and Cultures School of edicine raduate School of Science Faculty of Science School of Dentistry raduate School of athematics Faculty of athematics School of Pharmaceutical Sciences raduate School of Systems ife Sciences Faculty of edical Sciences School of ngineering raduate School of edical Sciences Faculty of Dental Science School of Design raduate School of Dental Science Faculty of Pharmaceutical Sciences School of Agriculture raduate School of Pharmaceutical Sciences Faculty of ngineering raduate School of ngineering Faculty of Design Faculty of Information Science and lectrical raduate School of Design ngineering raduate School of Information Science and Faculty of ngineering Sciences lectrical ngineering Interdisciplinary raduate School of ngineering Faculty of Agriculture Sciences -

Acknowledgments the Authors and Publisher Would Like to Thank the Following Teaching Professionals for Their Valuable Feedback During the Development of the Series
Turn the Tide on Plastic. Here’s How,” by Laura Parker: NGM, Jun 2018, 161-162 Adapted from “Mystery on Everest,” by Conrad Anker: NGM, Oct 1999, and “Out of Thin Air,” by David Roberts: NGA, Fall 1999, 167 Adapted from “Amelia Earhart,” by Virginia Morell: NGM, Jan 1998, and based on information from “Forensic Dogs Locate Spot Where Amelia Earhart May Have Died,” by Rachel Hartigan Shea: news.nationalgeographic.com NGK = National Geographic Kids NGM = National Geographic Magazine NGA = National Geographic Adventure Acknowledgments The Authors and Publisher would like to thank the following teaching professionals for their valuable feedback during the development of the series. Akiko Hagiwara, Tokyo University of Pharmacy and Life Sciences; Albert Lehner, University of Fukui; Alexander Cameron, Kyushu Sangyo University; Amira Traish, University of Sharjah; Andrés López, Colégio José Max León; Andrew Gallacher, Kyushu Sangyo University; Angelica Hernandez, Liceo San Agustin; Angus Painter, Fukuoka University; Anouchka Rachelson, Miami Dade College; Ari Hayakawa, Aoyama Gakuin University; Atsuko Otsuki, Senshu University; Ayako Hisatsune, Kanazawa Institute of Technology; Bogdan Pavliy, Toyama University of International Studies; Braden Chase, The Braden Chase Company; Brian J. Damm, Kanda Institute of Foreign Languages; Carol Friend, Mercer County Community College; Catherine Yu, CNC Language School; Chad Godfrey, Saitama Medical University; Cheng-hao Weng, SMIC Private School; Chisako Nakamura, Ryukoku University; Chiyo Myojin, Kochi -

The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension
Hypertension Research (2014) 37, 254–255 & 2014 The Japanese Society of Hypertension All rights reserved 0916-9636/14 www.nature.com/hr The Japanese Society of Hypertension Committee for Guidelines for the Management of Hypertension CHAIRPERSON Kazuaki SHIMAMOTO (Sapporo Medical University) WRITING COMMITTEE Katsuyuki ANDO (University of Tokyo) Ikuo SAITO (Keio University) Toshihiko ISHIMITSU (Dokkyo Medical University) Shigeyuki SAITOH (Sapporo Medical University) Sadayoshi ITO (Tohoku University) Kazuyuki SHIMADA (Jichi Medical University) Masaaki ITO (Mie University) Kazuaki SHIMAMOTO (Sapporo Medical University) Hiroshi ITOH (Keio University) Tatsuo SHIMOSAWA (University of Tokyo) Yutaka IMAI (Tohoku University) Hiromichi SUZUKI (Saitama Medical University) Tsutomu IMAIZUMI (Kurume University) Norio TANAHASHI (Saitama Medical University) Hiroshi IWAO (Osaka City University) Kouichi TAMURA (Yokohama City University) Shinichiro UEDA (University of the Ryukyus) Takuya TSUCHIHASHI (Steel Memorial Yahata Hospital) Makoto UCHIYAMA (Uonuma Kikan Hospital) Mitsuhide NARUSE (NHO Kyoto Medical Center) Satoshi UMEMURA (Yokohama City University) Koichi NODE (Saga University) Yusuke OHYA (University of the Ryukyus) Jitsuo HIGAKI (Ehime University) Katsuhiko KOHARA (Ehime University) Naoyuki HASEBE (Asahikawa Medical College) Hisashi KAI (Kurume University) Toshiro FUJITA (University of Tokyo) Naoki KASHIHARA (Kawasaki Medical School) Masatsugu HORIUCHI (Ehime University) Kazuomi KARIO (Jichi Medical University) Hideo MATSUURA (Saiseikai Kure Hospital) -

1. Japanese National, Public Or Private Universities
1. Japanese National, Public or Private Universities National Universities Hokkaido University Hokkaido University of Education Muroran Institute of Technology Otaru University of Commerce Obihiro University of Agriculture and Veterinary Medicine Kitami Institute of Technology Hirosaki University Iwate University Tohoku University Miyagi University of Education Akita University Yamagata University Fukushima University Ibaraki University Utsunomiya University Gunma University Saitama University Chiba University The University of Tokyo Tokyo Medical and Dental University Tokyo University of Foreign Studies Tokyo Geijutsu Daigaku (Tokyo University of the Arts) Tokyo Institute of Technology Tokyo University of Marine Science and Technology Ochanomizu University Tokyo Gakugei University Tokyo University of Agriculture and Technology The University of Electro-Communications Hitotsubashi University Yokohama National University Niigata University University of Toyama Kanazawa University University of Fukui University of Yamanashi Shinshu University Gifu University Shizuoka University Nagoya University Nagoya Institute of Technology Aichi University of Education Mie University Shiga University Kyoto University Kyoto University of Education Kyoto Institute of Technology Osaka University Osaka Kyoiku University Kobe University Nara University of Education Nara Women's University Wakayama University Tottori University Shimane University Okayama University Hiroshima University Yamaguchi University The University of Tokushima Kagawa University Ehime -

Kurume Region O Fukuoka Industrial Technology Center (FITC), V
Knowledge Clusters:InnovativeStage(Active) Knowledge Clusters:InnovativeStage(Active)K n o (Fiscal Year 2009-2013) w Cluster Headquarters Participating Research Organizations (Bold: Core Research Organization) l e ○President……………Wataru Aso Industry…GreenPeptide Co., Ltd., Cell Innovator, d International Science Technology Co.,LTd,., ASTEC Co.,LTD., g ○Vice President………Morikuni Eto e Life Sciences IT Environment Nanotech/Materials Kurume Cutting-edge Medical Research Cluster ○Project Director………Hiroshi Gushima Nipro Corporation, Dojindo Molecular Technologies, Inc. C l ○Chief Scientist (CS)……Kyogo Ito Academia…Kurume University, Kyushu University, u s Kyushu Sangyo University, Kyoto University, Kumamoto University, t ○Sub-Project Director……Akinobu Ohuchida (Doubles as Science and Technology Coordinator e Kitasato University, Kinki University, Hokkaido University, r responsible for pharmaceutical development safety) s : Hirosaki University, Teikyo University, I ○Vice-Chief Scientist……Yoshifumi Ikeda (Doubles as Science and Technology Coordinator n The Jikei University School of Medicine, and Tohoku University n responsible for pharmaceutical development) o Government…The Biotechnology and Food Research Institute (BFRI) and Kurume region v ○Human Resources Development Coordinator…Yuko Kato Chemical and Textile Industry Research Institute (CTRI) of a t i (Globalization Coordinator, Clinical Science and Technology Coordinator Assistant, etc.— three people.) Fukuoka Industrial Technology Center (FITC), v e Formation of a world-leading, cutting-edge -
Partnering Universities and Colleges List(As of January 1, 2021)
■Partnering Universities and Colleges list(As of January 1, 2021) No. Prefectures Name No. Prefectures Name No. Prefectures Name 1 Hokkaido Asahikawa Medical University 81 Fukushima Koriyama Women's University 161 Chiba Kameda College of Health Sciences 2 Hokkaido Otaru University of Commerce 82 Fukushima Higashi Nippon International University 162 Chiba Kawamura Gakuen Women's University 3 Hokkaido Obihiro University of Agriculture and Veterinary Medicine 83 Fukushima Iwaki Junior College 163 Chiba Kanda University of International Studies 4 Hokkaido Kitami Institute of Technology 84 Fukushima Koriyama Women's College 164 Chiba Keiai University 5 Hokkaido Hokkaido University of Education 85 Ibaraki Ibaraki University 165 Chiba International Budo University 6 Hokkaido Hokkaido University 86 Ibaraki Tsukuba University of Technology 166 Chiba Shumei University 7 Hokkaido Muroran Institute of Technology 87 Ibaraki University of Tsukuba 167 Chiba Shukutoku University 8 Hokkaido Sapporo Medical University 88 Ibaraki Ibaraki Prefectural University of Health Sciences 168 Chiba Josai International University 9 Hokkaido Sapporo City University 89 Ibaraki Ibaraki Christian University 169 Chiba Seitoku University 10 Hokkaido Asahikawa University 90 Ibaraki Tsukuba Gakuin University 170 Chiba Seiwa University 11 Hokkaido Sapporo Gakuin University 91 Ibaraki Tsukuba International University 171 Chiba Chiba Institute of Science 12 Hokkaido Sapporo International University 92 Ibaraki Tokiwa University 172 Chiba Chiba Keizai University 13 Hokkaido Sapporo -
How to Answer the Survey ◆ ○ This Survey Is Offered in Both Japanese and English, but the Contents Are the Same
◆ How to answer the survey ◆ ○ This survey is offered in both Japanese and English, but the contents are the same. Please fill out just one survey. ○ Please read the question and mark the appropriate answer option with a circle (〇). For answer options which indicate “Please answer question (A, B, etc.)” next to the option, please also answer the related question. ○ When you have completed the survey, please place it in the return envelope, and place it in a post box by Wednesday, December 27, 2017. Postage is not required. ○ Contact for inquiries on the survey: Hasegawa, Yamaguchi - International Policy Section, International Affairs Department, General Affairs & Planning Bureau, Fukuoka City Tel 092-711-4529 E-mail [email protected] ○ The results of this survey will be published in a way that prevents the identification of all respondents. ○ The results of the survey will be published on the Fukuoka City website. ○ The personal information collected in this survey will be handled according to the bylaws for personal information protection of Fukuoka City. About the respondent (you) 1. What is your country or region of origin? Please select 1 answer. 1. China 2. Vietnam 3. Nepal 4. Republic of Korea 5. Indonesia 6. Thailand 7. Taiwan 8. Bangladesh 9. Myanmar 10. Egypt 11. Malaysia 12. India 13. Sri Lanka 14. United States 15. Mongolia 16. Other (Specifically: ) 2. In what area are you currently residing? Please select 1 answer. 1. Higashi Ward, Fukuoka 2. Hakata Ward, Fukuoka 3. Chuo Ward, Fukuoka 4. Minami Ward, Fukuoka 5. Jonan Ward, Fukuoka 6. -

Business Environment in Kyushu
Business Environment in Kyushu October, 2012 Kyushu Bureau of Economy, Trade and Industry Direct investments into Japan from foreign countries generate a wide variety of benefits, such as the addition of high added-value to industries, creation of employment opportunities, stimulation of local economies, and enhancement of consumer interests. Such investments enhance Japan‟s entire economic growth potential and contribute to the revitalization of local economies. In this regard, they are important to our country. To attract foreign companies to Japan, we believe it is important to raise the attention of as many companies as possible, by disseminating and providing the country‟s regional information. This document explains the strong points of Kyushu as an excellent investment destination: its vicinity to Asia, wealth of industries such as automobiles and semiconductors, and research and development capacity. Table of Contents Table of Contents…………………………… 1 < I. Outline of Kyushu > (Appendices) Kyushu in Asia ………………………………2 Trade Structure in Kyushu ……………..21 - 22 Scale of Kyushu‟s Economy ……………..3 - 4 Main Foreign Companies in Kyushu‟s Transportation Infrastructure Kyushu ……………………………….....23 - 26 – Air Routes and Access to Major Profiles of Foreign Companies in Japanese Cities ………………….…......... 5 Kyushu ……………………………………....27 – Ports, Expressways and Railways ………. 6 Profiles of Kyushu Companies Allied with Foreign Companies ……………………28 < II. Kyushu‟s Industry, Research and Development> Economic Exchanges between Kyushu and Foreign Countries……………………………29