A Toast to an Enzyme
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Dehydrogenase Gene of Entamoeba Histolytica
Proc. Nad. Acad. Sci. USA Vol. 89, pp. 10188-10192, November 1992 Medical Sciences Cloning and expression of an NADP+-dependent alcohol dehydrogenase gene of Entamoeba histolytica (amebiasis/protozoan parasite/fermentation/inhibitors) AJIT KUMAR*, PEI-SHEN SHENtt, STEVEN DESCOTEAUX*, JAN POHL§, GORDON BAILEYt, AND JOHN SAMUELSON*1II *Department of Tropical Public Health, Harvard School of Public Health, Boston, MA 02115; tDepartment of Biochemistry, Morehouse School of Medicine, Atlanta, GA 30310; §Microchemical Facility, Emory University, Atlanta, GA 30322; and 'Department of Pathology, New England Deaconess Hospital, Boston, MA 02215 Communicated by Elkan Blout, June 29, 1992 (receivedfor review May 1, 1992) ABSTRACT Ethanol is the major metabolic product of NAD(P)+ so that the reducing equivalents are regenerated glucose fermentation by the protozoan parasite Entmoeba (4-7). Both NADP+-dependent and NAD+-dependent alco- histolytica under the anaerobic conditions found in the lumen hol dehydrogenase (ADH) activities have been identified in of the colon. Here an internal peptide sequence determined E. histolytica trophozoites (4-7). The NADP+-dependent from a major 39-kDa amoeba protein isolated by isoeLtric ADH (EhADHi; EC 1.1.1.2) was found to be composed of focusing followed by SDS/PAGE was used to clone the gene for four -30-kDa subunits, prefer secondary alcohols to primary the E. histolytica NADP+-dependent alcohol dehydrogenase alcohols, and be inhibited by the substrate analogue pyrazole (EhADH1; EC 1.1.1.2). The EhADHi clone had an open (7). The amoeba NAD+-dependent ADH (EC 1.1.1.1) is not reading frame that was 360 amino acids long and encoded a well-characterized (4). -

Relation Between the Recipe of Yeast Dough Dishes and Their Glycaemic Indices and Loads
foods Article Relation between the Recipe of Yeast Dough Dishes and Their Glycaemic Indices and Loads Ewa Raczkowska * , Karolina Ło´zna , Maciej Bienkiewicz, Karolina Jurczok and Monika Bronkowska Department of Human Nutrition, Faculty of Biotechnology and Food Sciences, Wrocław University of Environmental and Life Sciences, 51-630 Wroclaw, Poland * Correspondence: [email protected]; Tel.: +48-71-320-7726 Received: 23 July 2019; Accepted: 30 August 2019; Published: 1 September 2019 Abstract: The aim of the study was to evaluate the glycaemic indices (GI) and glycaemic loads (GL) of four food dishes made from yeast dough (steamed dumplings served with yoghurt, apple pancakes sprinkled with sugar powder, rolls with cheese and waffles with sugar powder), based on their traditional and modified recipes. Modification of the yeast dough recipe consisted of replacing wheat flour (type 500) with whole-wheat flour (type 2000). Energy value and the composition of basic nutrients were assessed for every tested dish. The study was conducted on 50 people with an average age of 21.7 1.1 years, and an average body mass index of 21.2 2.0 kg/m2. The GI of the analysed ± ± food products depended on the total carbohydrate content, dietary fibre content, water content, and energy value. Modification of yeast food products by replacing wheat flour (type 500) with whole-wheat flour (type 2000) contributed to the reduction of their GI and GL values, respectively. Keywords: glycaemic index; glycaemic load; yeast dough 1. Introduction In connection with the growing number of lifestyle diseases, consumers pay increasing attention to food, not only to food that have a better taste but also to food that help maintain good health. -

Kilimanjaro Sample Menu
Kilimanjaro Sample Menu DAY 1: Big tree Camp (Mti Mkubwa) Lunch box- Fruits Arrive at camp- Tea or coffee, biscuits, cashew nuts, popcorn Dinner- Starter: Cream of pumpkin soup with garlic bread Main course: Fried fish fillet served with boiled potatoes and green vegetables Dessert- Bananas, oranges Tea and coffee DAY 2: Shira One Camp Breakfast- Morning tea, coffee, juice Assorted fruits Toast with butter, honey and peanut butter Oatmeal, sausage or bacon, eggs, hash browns Lunch- Fried chicken Cheese sandwich served with cucumber Soup and fresh carrots and cucumber Fruit, chocolates Arrive at camp: Hot drinks, popcorn, peanuts and cakes Dinner- Plantain stew with kachumbari(red onion/tomato salad) and bread Spaghetti ragu, mashed potatoes, cabbage & vegetable salad Dessert – Banana fritters, tea, drinking chocolate. DAY 3: Shira Two Camp Breakfast- Morning tea, coffee, juice Omelet, eggs, toast Cornflakes with hot milk Assorted fruits Kilimanjaro Sample Menu Lunch- French toast with hard-boiled eggs Guacamole and cakes Juice, mangos, bananas, cashews Tea, coffee and drinking chocolate Arrive at camp: Hot drinks, mahamri (East African donuts) and biscuits Dinner- Fresh cream of vegetables soup with bread, Fried rice with meat and chapati (thick tortilla) Dessert- Scrambled eggs Pineapple flambé or mangos Tea or coffee DAY 4: Baranco Camp Breakfast- Morning tea, coffee, juice Assorted fruits Toast Oatmeal, scrambled eggs with cucumber and tomato slices Tea, coffee Lunch- Carrot soup with bread Potato salad with chicken Spaghetti -

Toaster Oven
840073000v02.qxd 6/29/01 1:58 PM Page 1 Toaster Oven Safety ............................................ 2 Parts and Features ...................... 5 To Toast ........................................ 6 To Bake.......................................... 8 To Broil .......................................... 8 Cleaning ........................................ 9 Troubleshooting ........................ 10 Customer Service in USA 1-800-851-8900 840073000 840073000v02.qxd 6/29/01 1:58 PM Page 2 15. Oversize foods or metal utensils must not be inserted in a IMPORTANT SAFEGUARDS toaster oven as they may create a fire or risk of electric shock. When using electrical appliances, basic safety precautions should 16. A fire may occur if the toaster oven is covered, touching or near always be followed, including the following: flammable material, including curtains, draperies, walls, and the 1. Read all instructions. like, when in operation. Do not store any item on top of the 2. Do not touch hot surfaces. Use handles or knobs. appliance when in operation, or before the appliance cools 3. To protect against electrical shock do not immerse cord, plug, down. or toaster oven in water or other liquid. 17. Extreme caution should be exercised when using containers 4. Close supervision is necessary when any appliance is used by constructed of other than metal or glass. or near children. 18. Do not store any materials, other than Hamilton Beach/ 5. Unplug from outlet when not in use and before cleaning. Allow Proctor-Silex, Inc. recommended accessories, in this oven. to cool before cleaning appliance and putting on or taking off 19. Do not place any of the following materials in the oven: paper, parts. cardboard, plastic, and the like. 6. -

Hot Sandwiches Burgers
SANDWICH BOARD MAKE IT DELUXE! INCLUDES: POTATOES, OR RICE, AND CHOICE OF COLESLAW, OR CUP OF SOUP 3.25 | FRIES ONLY 2.25 WE CAN MAKE ANY SANDWICH ON PITA BREAD OR WRAP 0.75 CONEY ISLAND 2.55 Hot dog topped with chili and onions CORNED BEEF & SWISS 6.50 Grilled on Rye bread REUBEN 6.95 BURGERS Our lean, juicy corned beef with Swiss cheese and sauerkraut, on grilled Rye bread ¹/³ lb. Angus Ground Beef, Broiled and Served with Lettuce, TURKEY REUBEN 6.75 Tomato, and Pickle Garnish Our fresh turkey with Swiss cheese and sauerkraut, Make It a Double Burger add 3.50 on grilled Rye bread HAMBURGER COLD HAM OR TURKEY SANDWICH 4.95 5.95 CHEESEBURGER Hand cut fresh, ham or all white meat turkey, 5.50 served with lettuce, tomato and mayo BACON CHEESEBURGER 5.95 MUSHROOM SWISS BURGER 5.95 SLIM HEO 6.95 Grilled ham, Swiss cheese with lettuce and tomato HEO’S BURGER 7.95 ½ lb. hamburger with bacon, mushrooms, grilled FRENCH DIP 6.95 onions, and Cheddar cheese Lean, thin sliced roast beef, served on a French Roll with Au Jus PAULI BURGER 6.50 TUNA SANDWICH 6.25 1/3lb. hamburger with Cheddar cheese, bacon & Albacore tuna served on your choice of toast with lettuce and tomato sunny side up egg CRISPY CHICKEN SANDWICH 6.95 Breaded chicken breast served with Swiss cheese, lettuce and tomato B.L.T. 5.75 Generous portion of bacon with mayo, lettuce and tomato TRIPLE DECKER CLUBS GRILLED CHICKEN PITA 5.95 TRIPLE DECKER CLUB 6.95 Breast of chicken with lettuce and tomato Choice of ham or turkey with bacon, Swiss GYROS SANDWICH 6.25 cheese, lettuce, tomato -

Is There a Role for Ethanol Assay Using Alcohol Dehydrogenase in a Basic Drugs and Alcohol Screen of Blood Following Routine Autopsies?
Journal of Clinical Pathology and Forensic Medicine Vol. 3(2), pp. 17-19, December 2012 Available online at http://www.academicjournals.org/JCPFM DOI: 10.5897/JCPFM12.003 ISSN 2I41-2405 ©2012 Academic Journals Short Communication Is there a role for ethanol assay using alcohol dehydrogenase in a basic drugs and alcohol screen of blood following routine autopsies? William Tormey 1,2 and Tara Moore 3 1Biomedical Sciences, University of Ulster Cromore Road, Coleraine, BT52 1SA, Northern Ireland. 2Department of Chemical Pathology, Beaumont Hospital, Dublin 9, Ireland. 3Biomedical Sciences, University of Ulster, Cromore Road, Coleraine, BT52 1SA, Northern Ireland. Accepted 22 March, 2012 The external examination system of determining the cause of death as operated in Dundee, Scotland is controversial. The addition of a blood or urine specimen for drugs and alcohol processed by hospital autoanalysers will reduce error in death certification. This is even more convenient to organise with a shorter turn-around time than imaging by computed tomography (CT) or magnetic resonance imaging (MRI). These measures may reduce the autopsy rate and ameliorate the distress of families of the deceased. Key words: Autopsy, alcohol, death. INTRODUCTION For over a decade, there has been controversy over the dehydrogenase and the back reaction nicotinamide appropriateness of the high rate of coroner’s autopsies adenine dinucleotide (NAD) to NADH measured (Pounder, 2000; Rutty et al., 2001). Advocacy of the spectrophotometrically at 340 nm on the Olympus 5400 “view and grant” system of issuing the cause of death as autoanalyser for the analysis of alcohol. The data sheet operated in Dundee, Scotland has been challenged and claims that the assay can accurately quantitate alcohol imaging is suggested as a partial answer to lessen the concentrations within the range of 10 to 600 mg/dl. -

How Is Alcohol Metabolized by the Body?
Overview: How Is Alcohol Metabolized by the Body? Samir Zakhari, Ph.D. Alcohol is eliminated from the body by various metabolic mechanisms. The primary enzymes involved are aldehyde dehydrogenase (ALDH), alcohol dehydrogenase (ADH), cytochrome P450 (CYP2E1), and catalase. Variations in the genes for these enzymes have been found to influence alcohol consumption, alcohol-related tissue damage, and alcohol dependence. The consequences of alcohol metabolism include oxygen deficits (i.e., hypoxia) in the liver; interaction between alcohol metabolism byproducts and other cell components, resulting in the formation of harmful compounds (i.e., adducts); formation of highly reactive oxygen-containing molecules (i.e., reactive oxygen species [ROS]) that can damage other cell components; changes in the ratio of NADH to NAD+ (i.e., the cell’s redox state); tissue damage; fetal damage; impairment of other metabolic processes; cancer; and medication interactions. Several issues related to alcohol metabolism require further research. KEY WORDS: Ethanol-to acetaldehyde metabolism; alcohol dehydrogenase (ADH); aldehyde dehydrogenase (ALDH); acetaldehyde; acetate; cytochrome P450 2E1 (CYP2E1); catalase; reactive oxygen species (ROS); blood alcohol concentration (BAC); liver; stomach; brain; fetal alcohol effects; genetics and heredity; ethnic group; hypoxia The alcohol elimination rate varies state of liver cells. Chronic alcohol con- he effects of alcohol (i.e., ethanol) widely (i.e., three-fold) among individ- sumption and alcohol metabolism are on various tissues depend on its uals and is influenced by factors such as strongly linked to several pathological concentration in the blood T chronic alcohol consumption, diet, age, consequences and tissue damage. (blood alcohol concentration [BAC]) smoking, and time of day (Bennion and Understanding the balance of alcohol’s over time. -

Our Twist on the Classic South Indian Lentil Soup. We're Cooking up Lentils
35 Our twist on the classic South Indian lentil soup. We’re cooking up lentils and 15 acorn squash, in a creamy curry stew. With fresh apple chunks and bell peppers, 1 Whisk it’s fresh and crunchy and totally delicious. Served with warm naan. EQUIPMENT Health snapshot per serving – 535 Calories, 20g Fat, 87g Carbs, 14g Protein, 12 Saucepan with a Cover Freestyle Points Large Skillet Have questions? The dinner hotline is standing by from 5 to 8 pm at 773.916.6339. FROM YOUR PANTRY Olive Oil Salt & Pepper 7 MEEZ CONTAINERS Mirepoix Lentils Broth Starter Acorn Squash Apple Bell Peppers Naan INGREDIENTS: Naan Bread, Apple, Acorn Squash, Bell Peppers, Coconut Milk, Carrot, Tomato, Pumpkin, Lentils, Celery, Onion, Garlic, Ginger, Coriander, Garam Masala, Thyme, Curry Powder, Cinnamon, Vegetable Stock, Lime, Kosher Salt, Black Pepper. 1. Cook the Broth Heat 3 Tbsp olive oil in a saucepan over medium low heat. When the oil is hot, add the Mirepoix and cook until the onions start to turn golden brown and the mix is aromatic, about 2 to 3 minutes. Turn the heat to high and add the Lentils, Broth Starter, and Acorn Squash along with 2 cups of water. Bring to a boil, making sure to scrape the bottom for the tasty bits. Lower the heat to medium, cover, and simmer until the lentils soften and their texture is creamy, about 20 minutes. 2. Add Apples and Bell Peppers When the soup has about 5 minutes left to simmer, cut each Apple into quarters and then dice each quarter into ½” cubes. -

Yeast Bread Handling and Storage
Yeast Bread Handling and Storage Holding baked bread: To prevent staling or hardening (see Note below) • Never refrigerate unless filled with custard or meat. • Never hold in cool or drafty place even if wrapped. • Staling occurs most rapidly between 20 and 50º F. • Cool products to an interior temperature of 90º F., then wrap. • Hold products at 70-95º F. and under 110º F. Crusty breads: • Store in packaging with end open or in breathable plastic bags. • Use the day they are made or freeze immediately after cooling to interior temperature of 90º F. Refresh and warm breads just prior to serving: • Warm bread before serving if it is 60º F. or colder • Place crusty breads/rolls in very hot oven, unwrapped, for 2 to 5 minutes. • Soft rolls may be wrapped/covered and placed in a very hot oven for 5 minutes to warm, or placed in a food warmer at 95–105º F. • Do not warm breads in microwave—they are too easily over-heated and will harden more rapidly. • Keep warm breads covered as long as possible; hold in a 95º F. place. Freezing: Freezing bread the day it is baked will mean the bread is the same as day-old when thawed. (Holding bread at 70-95º F. in a draft free place for 24 hours will be the same result.) • Freeze bread if it will be two days before use. • Wrap in air-tight, freezer-suitable packaging. Label with “date in” “date out” information. • Freeze and maintain frozen rolls or breads at 0º F. • Breads will keep frozen for several weeks. -
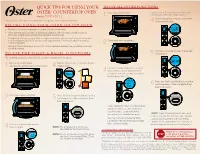
Quick Tips for Using Your Oster® Countertop Oven
QUICK TIPS FOR USING YOUR TO USE ALL OTHER FUNCTIONS OSTER® COUNTERTOP OVEN Place food on Wire Rack. Follow steps 4-5 to modify preset time and temperature. Otherwise, skip to step 6. Model: TSSTTVDFL1 1 Turn the Temperature Knob to select the Refer to your Oven Manual for Important Safeguards and cleaning instructions. 4 desired temperature. BEFORE USING YOUR COUNTERTOP OVEN • Remove any stickers and papers in and on the countertop oven. • Clean the wire rack, broil rack and baking/cookie pan with hot water, a small amount of dishwashing liquid and a nonabrasive sponge or cleaning pad. ** During initial start up, you may detect a slight smell and/or smoke. Don’t worry this is normal. Turn the temperature to 450º and let run for approximately 5 minutes to allow the smell or Close glass door completely. smoke to dissipate. 2 Warning: Make sure there is a 6-inch (15.24cm) clearance between the countertop oven and any other surface. Turn the Time Knob to select the desired TO USE THE TOAST & BAGEL FUNCTIONS 5 cooking time. We simplified toasting for more precise, consistent results with less hassle. Place food on Wire Rack. Turn the Time Knob to select the desired 1 (Use bottom rack position for best results.) 4 shade setting. Turn the Function Knob to Pizza, Bake, 3 Broil, Cookies, Roast, Warm, Defrost or Reheat. The selected function will be highlighted on the LCD. Press the Start/Cancel Button to start the 6 cooking process. When complete, three beeps will sound. Close glass door completely. Press the Start/Cancel Button to start the 2 5 toasting process. -

1 Here Are the Quiz 4 Questions You Will Answer Online Using the Quiz
Chemistry 6720, quiz 4 handout Here are the quiz 4 questions you will answer online using the quiz tool in canvas instructure. Some of the questions deal with citric acid cycle enzymes that use mechanistic strategies covered in the lectures. Since we did not cover all of those specific enzymes in lecture, be sure to review the citric acid cycle ahead of taking the quiz online so you can answer the appropriate questions regarding mechanistic strategies/themes for the enzymes. In doing so, practice drawing mechanisms for each enzyme of the citric acid cycle. You will probably need to look up the mechanisms for isocitrate dehydrogenase, aconitase, succinate dehydrogenase, and succinyl-CoA synthetase in a general biochemistry text. I will not be asking specific details of the mechanisms for those 4 enzymes, but you will still need to be generally familiar with how they operate to answer a few of the questions below. You do not need to know the chemistry of FAD reduction for the quiz. Be sure to note the quiz deadline in canvas to be sure you get your answers submitted by the deadline. 1. Shown at the right is a Fischer projection for D-glucose. Use the strategy described in the lecture to assign the configurations (R or S) of the chiral centers at C2, C3, C4, and C5. 2. Using the structures shown to the right, assign the methyl groups of isopropanol, and the hydrogens at C2 and C3 of succinate, as either ProR or ProS. Use the strategy covered in lecture to guide you in making the assignments. -

Original Article Compensatory Upregulation of Aldo-Keto Reductase 1B10 to Protect Hepatocytes Against Oxidative Stress During Hepatocarcinogenesis
Am J Cancer Res 2019;9(12):2730-2748 www.ajcr.us /ISSN:2156-6976/ajcr0097527 Original Article Compensatory upregulation of aldo-keto reductase 1B10 to protect hepatocytes against oxidative stress during hepatocarcinogenesis Yongzhen Liu1, Jing Zhang1, Hui Liu1, Guiwen Guan1, Ting Zhang1, Leijie Wang1, Xuewei Qi1, Huiling Zheng1, Chia-Chen Chen1, Jia Liu1, Deliang Cao2, Fengmin Lu3, Xiangmei Chen1 1State Key Laboratory of Natural and Biomimetic Drugs, Department of Microbiology and Infectious Disease Center, School of Basic Medical Sciences, Peking University Health Science Center, Beijing 100191, P. R. China; 2Department of Medical Microbiology, Immunology and Cell Biology, Simmons Cancer Institute at Southern Illinois University School of Medicine, 913 N, Rutledge Street, Springfield, IL 62794, USA; 3Peking University People’s Hospital, Peking University Hepatology Institute, Beijing 100044, P. R. China Received May 26, 2019; Accepted November 15, 2019; Epub December 1, 2019; Published December 15, 2019 Abstract: Aldo-keto reductase 1B10 (AKR1B10), a member of aldo-keto reductase superfamily, contributes to detox- ification of xenobiotics and metabolization of physiological substrates. Although increased expression of AKR1B10 was found in hepatocellular carcinoma (HCC), the role of AKR1B10 in the development of HCC remains unclear. This study aims to illustrate the role of AKR1B10 in hepatocarcinogenesis based on its intrinsic oxidoreduction abilities. HCC cell lines with AKR1B10 overexpression or knockdown were treated with doxorubicin or hydrogen peroxide to determinate the influence of aberrant AKR1B10 expression on cells’ response to oxidative stress. Using Akr1b8 (the ortholog of human AKR1B10) knockout mice, diethylnitrosamine (DEN) induced liver injury, chronic inflammation and hepatocarcinogenesis were explored. Clinically, the pattern of serum AKR1B10 relevant to disease progres- sion was investigated in a patient cohort with chronic hepatitis B (n=30), liver cirrhosis (n=30) and HCC (n=40).