Spectroscopic and Functional Modeling Studies of Vanadium Bromoperoxidase
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

On the Regiospecificity of Vanadium Bromoperoxidase
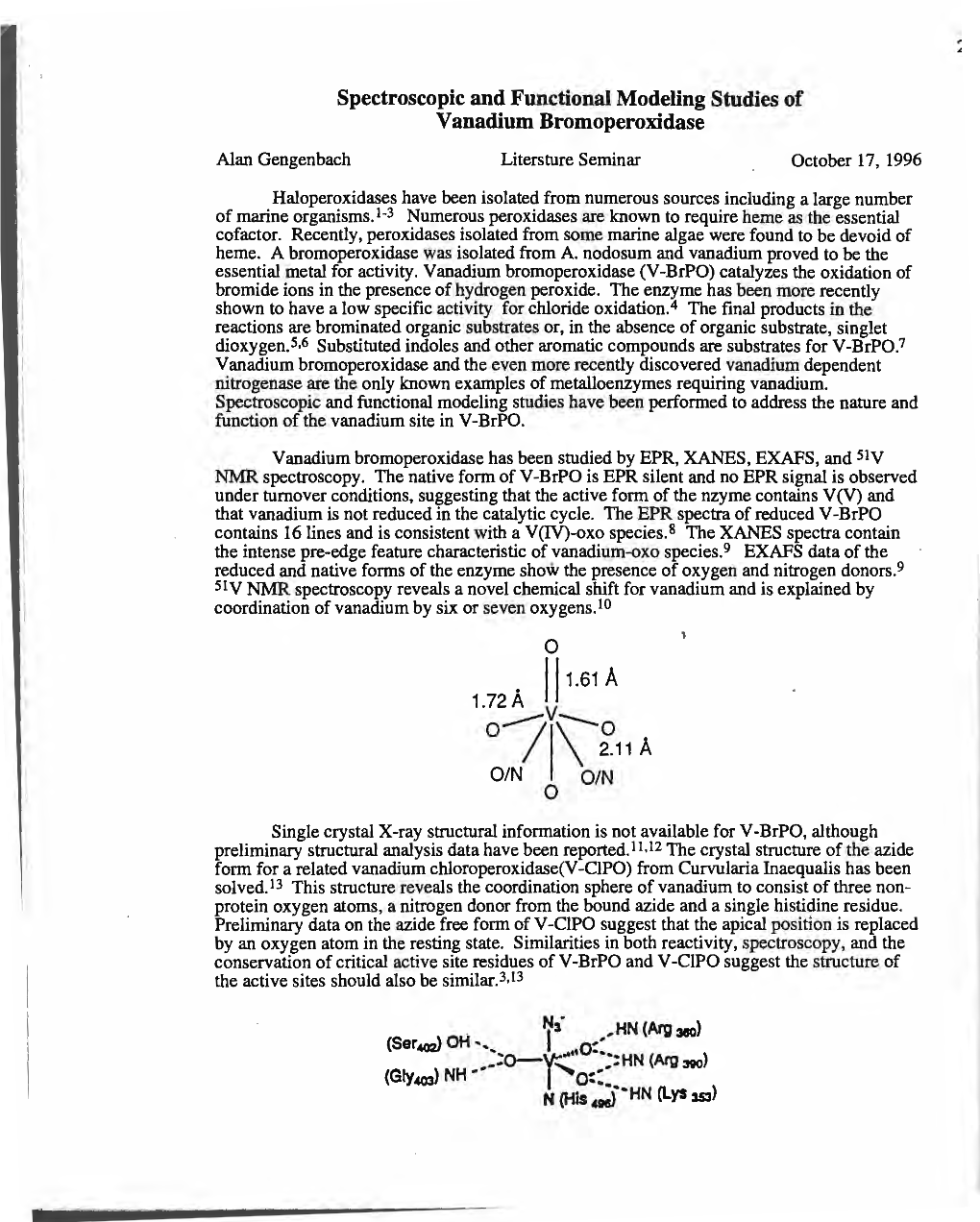
J. Am. Chem. Soc. 2001, 123, 3289-3294 3289 On the Regiospecificity of Vanadium Bromoperoxidase Jennifer S. Martinez, Georgia L. Carroll, Richard A. Tschirret-Guth, Gereon Altenhoff, R. Daniel Little, and Alison Butler* Contribution from the Department of Chemistry and Biochemistry, UniVersity of California, Santa Barbara, California 93106-9510 ReceiVed December 5, 2000 Abstract: Vanadium haloperoxidase enzymes catalyze the oxidation of halide ions by hydrogen peroxide, producing an oxidized intermediate, which can halogenate an organic substrate or react with a second equivalent of hydrogen peroxide to produce dioxygen. Haloperoxidases are thought to be involved in the biogenesis of halogenated natural products isolated from marine organisms, including indoles and terpenes, of which many are selectively oxidized or halogenated. Little has been shown concerning the ability of the marine haloperoxidases to catalyze regioselective reactions. Here we report the regiospecific bromoperoxidative oxidation of 1,3-di-tert-butylindole by V-BrPO from the marine algae Ascophyllum nodosum and Corallina officinalis. Both enzymes catalyze the regiospecific oxidation of 1,3-di-tert-butylindole in a reaction requiring - both H2O2 and Br as substrates, but which produce the unbrominated 1,3-di-tert-butyl-2-indolinone product exclusively, in near quantitative yield (i.e. one H2O2 consumed per product). By contrast, reactions with the - controlled addition of aqueous bromine solution (HOBr ) Br2 ) Br3 ) produce three monobromo and one dibromo-2-indolinone products, all of which differ from the V-BrPO-catalyzed product. Further, reactivities of 1,3-di-tert-butyl-2-indolinone with both aqueous bromine and V-BrPO differ significantly and shed light onto the possible nature of the oxidizing intermediate. -

A High-Throughput Screening System Based on Droplet Microfluidics For
molecules Article A High-Throughput Screening System Based on Droplet Microfluidics for Glucose Oxidase Gene Libraries Radivoje Prodanovi´c 1,2,* , W. Lloyd Ung 2, Karla Ili´c Đurđi´c 1 , Rainer Fischer 3, David A. Weitz 2 and Raluca Ostafe 4 1 Faculty of Chemistry, University of Belgrade, Studentski trg 12, 11000 Belgrade, Serbia; [email protected] 2 Department of Physics, School of Engineering and Applied Sciences, Harvard University, Cambridge, MA 02138, USA; [email protected] (W.L.U.); [email protected] (D.A.W.) 3 Departments of Biological Sciences and Chemistry, Purdue University, 207 S. Martin Jischke Dr., West Lafayette, IN 47907, USA; fi[email protected] 4 Purdue Institute of Inflammation, Immunology and Infectious Disease, Molecular Evolution, Protein Engineering and Production, Purdue University, 207 S. Martin Jischke Dr., West Lafayette, IN 47907, USA; [email protected] * Correspondence: [email protected]; Tel.: +38-111-333-6660 Academic Editors: Goran T. Vladisavljevi´cand Guido Bolognesi Received: 20 April 2020; Accepted: 15 May 2020; Published: 22 May 2020 Abstract: Glucose oxidase (GOx) is an important industrial enzyme that can be optimized for specific applications by mutagenesis and activity-based screening. To increase the efficiency of this approach, we have developed a new ultrahigh-throughput screening platform based on a microfluidic lab-on-chip device that allows the sorting of GOx mutants from a saturation mutagenesis library expressed on the surface of yeast cells. GOx activity was measured by monitoring the fluorescence of water microdroplets dispersed in perfluorinated oil. The signal was generated via a series of coupled enzyme reactions leading to the formation of fluorescein. -

New Automatically Built Profiles for a Better Understanding of the Peroxidase Superfamily Evolution
University of Geneva Practical training report submitted for the Master Degree in Proteomics and Bioinformatics New automatically built profiles for a better understanding of the peroxidase superfamily evolution presented by Dominique Koua Supervisors: Dr Christophe DUNAND Dr Nicolas HULO Laboratory of Plant Physiology, Dr Christian J.A. SIGRIST University of Geneva Swiss Institute of Bioinformatics PROSITE group. Geneva, April, 18th 2008 Abstract Motivation: Peroxidases (EC 1.11.1.x), which are encoded by small or large multigenic families, are involved in several important physiological and developmental processes. These proteins are extremely widespread and present in almost all living organisms. An important number of haem and non-haem peroxidase sequences are annotated and classified in the peroxidase database PeroxiBase (http://peroxibase.isb-sib.ch). PeroxiBase contains about 5800 peroxidase sequences classified as haem peroxidases and non-haem peroxidases and distributed between thirteen superfamilies and fifty subfamilies, (Passardi et al., 2007). However, only a few classification tools are available for the characterisation of peroxidase sequences: InterPro motifs, PRINTS and specifically designed PROSITE profiles. However, these PROSITE profiles are very global and do not allow the differenciation between very close subfamily sequences nor do they allow the prediction of specific cellular localisations. Due to the rapid growth in the number of available sequences, there is a need for continual updates and corrections of peroxidase protein sequences as well as for new tools that facilitate acquisition and classification of existing and new sequences. Currently, the PROSITE generalised profile building manner and their usage do not allow the differentiation of sequences from subfamilies showing a high degree of similarity. -

Halogenation Enzymes in Bacteria Associated with the Red-Banded Acorn Worm, Ptychodera Jamaicensis" (2011)
San Jose State University SJSU ScholarWorks Master's Theses Master's Theses and Graduate Research Fall 2011 Halogenation Enzymes in Bacteria Associated with the Red- banded Acorn Worm, Ptychodera jamaicensis Milena May Lilles San Jose State University Follow this and additional works at: https://scholarworks.sjsu.edu/etd_theses Recommended Citation Lilles, Milena May, "Halogenation Enzymes in Bacteria Associated with the Red-banded Acorn Worm, Ptychodera jamaicensis" (2011). Master's Theses. 4099. DOI: https://doi.org/10.31979/etd.snbx-gv6x https://scholarworks.sjsu.edu/etd_theses/4099 This Thesis is brought to you for free and open access by the Master's Theses and Graduate Research at SJSU ScholarWorks. It has been accepted for inclusion in Master's Theses by an authorized administrator of SJSU ScholarWorks. For more information, please contact [email protected]. HALOGENATION ENZYMES IN BACTERIA ASSOCIATED WITH THE RED- BANDED ACORN WORM, PTYCHODERA JAMAICENSIS A Thesis Presented to The Faculty of the Department of Biology San José State University In Partial Fulfillment of the Requirements for the Degree Master of Science by Milena M. Lilles December 2011 © 2011 Milena M. Lilles ALL RIGHTS RESERVED The Designated Thesis Committee Approves the Thesis Titled HALOGENATION ENZYMES IN BACTERIA ASSOCIATED WITH THE RED- BANDED ACORN WORM, PTYCHODERA JAMAICENSIS by Milena M. Lilles APPROVED FOR THE DEPARTMENT OF BIOLOGY SAN JOSÉ STATE UNIVERSITY December 2011 Dr. Sabine Rech Department of Biology Dr. Brandon White Department of Biology Dr. Roy Okuda Department of Chemistry ABSTRACT HALOGENATION ENZYMES IN BACTERIA ASSOCIATED WITH THE RED- BANDED ACORN WORM, PTYCHODERA JAMAICENSIS by Milena M. Lilles Organohalogens have diverse biological functions in the environment. -

A Comparative Review on the Catalytic Mechanism of Nonheme Iron Hydroxylases and Halogenases
Review A Comparative Review on the Catalytic Mechanism of Nonheme Iron Hydroxylases and Halogenases Amy Timmins and Sam P. de Visser * Manchester Institute of Biotechnology and School of Chemical Engineering and Analytical Science, The University of Manchester, 131 Princess Street, Manchester M1 7DN, UK; [email protected] * Correspondence: [email protected]; Tel.: +44-161-306-4882 Received: 17 July 2018; Accepted: 30 July 2018; Published: 31 July 2018 Abstract: Enzymatic halogenation and haloperoxidation are unusual processes in biology; however, a range of halogenases and haloperoxidases exist that are able to transfer an aliphatic or aromatic C–H bond into C–Cl/C–Br. Haloperoxidases utilize hydrogen peroxide, and in a reaction with halides (Cl−/Br−), they react to form hypohalides (OCl−/OBr−) that subsequently react with substrate by halide transfer. There are three types of haloperoxidases, namely the iron-heme, nonheme vanadium, and flavin-dependent haloperoxidases that are reviewed here. In addition, there are the nonheme iron halogenases that show structural and functional similarity to the nonheme iron hydroxylases and form an iron(IV)-oxo active species from a reaction of molecular oxygen with α- ketoglutarate on an iron(II) center. They subsequently transfer a halide (Cl−/Br−) to an aliphatic C–H bond. We review the mechanism and function of nonheme iron halogenases and hydroxylases and show recent computational modelling studies of our group on the hectochlorin biosynthesis enzyme and prolyl-4-hydroxylase as examples of nonheme iron halogenases and hydroxylases. These studies have established the catalytic mechanism of these enzymes and show the importance of substrate and oxidant positioning on the stereo-, chemo- and regioselectivity of the reaction that takes place. -

X-Ray Fluorescence Analysis Method Röntgenfluoreszenz-Analyseverfahren Procédé D’Analyse Par Rayons X Fluorescents
(19) & (11) EP 2 084 519 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: G01N 23/223 (2006.01) G01T 1/36 (2006.01) 01.08.2012 Bulletin 2012/31 C12Q 1/00 (2006.01) (21) Application number: 07874491.9 (86) International application number: PCT/US2007/021888 (22) Date of filing: 10.10.2007 (87) International publication number: WO 2008/127291 (23.10.2008 Gazette 2008/43) (54) X-RAY FLUORESCENCE ANALYSIS METHOD RÖNTGENFLUORESZENZ-ANALYSEVERFAHREN PROCÉDÉ D’ANALYSE PAR RAYONS X FLUORESCENTS (84) Designated Contracting States: • BURRELL, Anthony, K. AT BE BG CH CY CZ DE DK EE ES FI FR GB GR Los Alamos, NM 87544 (US) HU IE IS IT LI LT LU LV MC MT NL PL PT RO SE SI SK TR (74) Representative: Albrecht, Thomas Kraus & Weisert (30) Priority: 10.10.2006 US 850594 P Patent- und Rechtsanwälte Thomas-Wimmer-Ring 15 (43) Date of publication of application: 80539 München (DE) 05.08.2009 Bulletin 2009/32 (56) References cited: (60) Divisional application: JP-A- 2001 289 802 US-A1- 2003 027 129 12164870.3 US-A1- 2003 027 129 US-A1- 2004 004 183 US-A1- 2004 017 884 US-A1- 2004 017 884 (73) Proprietors: US-A1- 2004 093 526 US-A1- 2004 235 059 • Los Alamos National Security, LLC US-A1- 2004 235 059 US-A1- 2005 011 818 Los Alamos, NM 87545 (US) US-A1- 2005 011 818 US-B1- 6 329 209 • Caldera Pharmaceuticals, INC. US-B2- 6 719 147 Los Alamos, NM 87544 (US) • GOLDIN E M ET AL: "Quantitation of antibody (72) Inventors: binding to cell surface antigens by X-ray • BIRNBAUM, Eva, R. -

X-Ray Structure of a Vanadium-Containing Enzyme
Proc. Natl. Acad. Sci. USA Vol. 93, pp. 392-396, January 1996 Biochemistry X-ray structure of a vanadium-containing enzyme: Chloroperoxidase from the fungus Curvularia inaequalis (halogenation/bromoperoxidase/crystallography/coordination chemistry) ALBRECHT MESSERSCHMIDT* AND RON WEVERt *Max Planck Institute of Biochemistry, D-82152 Martinsried, Germany; and tE. C. Slater Institute, Department of Biochemistry, University of Amsterdam, 1018 TV Amsterdam, The Netherlands Communicated by Robert Huber, Max-Planck-Institut fuir Biochemie, Martinsried, Germany, August 7, 1995 (received for review June 3, 1995) ABSTRACT The chloroperoxidase (EC 1.11.1.-) from the Table 1. Data collection and phasing statistics fungus Curvularia inaequalis belongs to a class of vanadium enzymes that oxidize halides in the presence of hydrogen Parameter Native APWO APHG peroxide to the corresponding hypohalous acids. The 2.1 A Diffraction data crystal structure (R = 20%) of an azide chloroperoxidase Resolution 2.1 A 2.3 A 2.3 A complex reveals the geometry of the catalytic vanadium Total observations 132,089 103,740 101,456 center. Azide coordinates directly to the metal center, result- Unique reflections 42,197 32,289 31,231 ing in a structure with azide, three nonprotein oxygens, and Completeness (%)* 98.9/96.8 99.6/98.2 96.4/66.3 a histidine as ligands. In the native state vanadium will be Rmerget 0.094 0.084 0.078 bound as hydrogen vanadate(V) in a trigonal bipyramidal RFI 0.056 0.069 0.052 coordination with the metal coordinated to three oxygens in MIRAS phasing the equatorial plane, to the OH group at one apical position, Number of sites 1 1 and to the e2 nitrogen of a histidine at the other apical Riso (20 to 3.0 A)§ 0.110 0.078 position. -

Dissertation
1. Synthesis and Characterization of a Vanadium Based Transition State Mimic for Organophosphate Hydrolase 2. Catalytic Oxidation of Thioanisole and 2-Chloroethyl Ethyl Sulfide Using Resin Bead Supported Oxoperoxidovanadium(V) Complexes By Efram Goldberg Bachelor of Arts, Florida Atlantic University Masters of Science, Florida Institute of Technology A dissertation submitted to the Department of Biomedical and Chemical Engineering and Sciences and Florida Institute of Technology in partial fulfillment Of the requirements for the degree Doctor of Philosophy in Chemistry Melbourne, Florida December 2018 1. Synthesis and Characterization of a Vanadium Based Transition State Mimic for Organophosphate Hydrolase 2. Catalytic Oxidation of Thioanisole and 2-Chloroethyl Ethyl Sulfide Using Resin Bead Supported Oxoperoxidovanadium(V) Complexes A dissertation by Efram Goldberg Approved as to style and content __________________________________________ D. Andrew Knight, Ph.D., Committee Chairperson Professor and Chemistry Program Chair, Biomedical and Chemical Engineering and Sciences __________________________________________ Rudolf J. Wehmschulte, Dr. rer. nat. Professor, Biomedical and Chemical Engineering and Sciences __________________________________________ Nasri Nesnas, Ph.D. Professor, Biomedical and Chemical Engineering and Sciences __________________________________________ Gordon Patterson, Ph.D. Professor, School of Arts and Communication __________________________________________ Ted Conway, Ph.D. Professor and Department Head, Biomedical -

Marine Haloperoxidases
chem. Rev. 1998, 93, 1937-1944 Marine Haloperoxidases Alison Butler’ and J. V. Walker oepemnmt of ~%~II/SIIY. (~vasllyof cawom$. anta &&am. CaBlanh, 93106 Reodved A@ 20, 1993 (Revlsed Manusmt Racslved May 20, 1993) Contents I. Introduction 1937 A. Halogenated Compounds in the Marine 1937 Envlronment p: ? E. Marine Habperoxidases 1938 6 1. Types of Marine Haioperoxidases 1938 8. 2. Standard Assay of Habperoxidase 1939 Activity 11. Vanadium Bromoperoxidase 1939 A. Characterlstics of V-ErPO 1939 1. Isoiatlon and Purlflcation of VgrpO 1939 2. The Vanadium Sne 1939 .... .. ...:.;.. .. E. Reactivity Of V-ErpO 1940 . :. .. :. [.. 1. Halogenation and HalldeAssisted 1940 AII~~utler received her &A. from Reed College in 1977 and her Disproportionation of Hydrogen P~.D.from the University of California. San Diego. in 1962. Alter Peroxide pwMoaaal stays at UC Los Angeles and Caitech. she joked the 2. Specific Activltles 1941 faculty at UC Santa Barbara in 1986. She is a recent reciplent of an American Cancer Society. Junior Faculty Research Award, 1941 3. Enzyme Kinetics an Alfred P. Sbn Foundatlon Fellowship, and the UCSE PIOUS 4. Peroxide Selectivity 1941 TeacherlSchoiar Award. Her research interestsarein bioinaganic 5. Mechanistic Considerations 1941 chemistry and metalloblochemistry. including that of the marlne environment. C. Stability of V-ErPO 1942 D. Comment on the Putative Non-i-bma Iron 1942 Eromoperoxidases 111. Fell“ Bromoperoxidase 1942 A. Characteristics of FeHeme 1942 Eromoperoxbse E. Reactivity of FeHeme Bromoperoxidase 1942 1. Biosynthetic Investigations 1943 2. Other FeHema Haloperoxidases 1943 1V. Future Directions 1944 I. Introductlon A. Halogenated Compounds in the Msrlne Environment Jenylalne Vanessa Walker was born in St. -

Analytical Element and Method for The
Europäisches Patentamt *EP000848819B1* (19) European Patent Office Office européen des brevets (11) EP 0 848 819 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.7: G01N 33/543, C12Q 1/28 of the grant of the patent: 26.04.2000 Bulletin 2000/17 (86) International application number: PCT/US96/13270 (21) Application number: 96928884.4 (87) International publication number: (22) Date of filing: 16.08.1996 WO 97/09619 (13.03.1997 Gazette 1997/12) (54) ANALYTICAL ELEMENT AND METHOD FOR THE DETERMINATION OF A SPECIFIC BINDING LIGAND USING A VANADIUM BROMOPEROXIDASE AS A SIGNAL-GENERATING ENZYME ANALYTISCHES ELEMENT UND VERFAHREN ZUR BESTIMMUNG EINES SPEZIFISCHEN BINDENDEN LIGANDEN UNTER VERWENDUNG EINER VANADIN-BROMPEROXIDASE ALS EIN SIGNALGENERIERENDES ENZYM ELEMENT ET PROCEDE ANALYTIQUE DE DETERMINATION D’UN LIGAND SPECIFIQUE DE LIAISON UTILISANT LA BROMOPEROXYDASE DE VANADIUM COMME ENZYME GENERATRICE DE SIGNAUX (84) Designated Contracting States: • GROULX, Sarah, Fingar AT BE CH DE DK ES FI FR GB GR IE IT LI LU MC Ontario, NY 14519 (US) NL PT SE • KOPCIENSKI, Martha, Miller Designated Extension States: Spencerport, NY 14559 (US) SI (74) Representative: Mercer, Christopher Paul (30) Priority: 01.09.1995 US 523042 Carpmaels & Ransford 43, Bloomsbury Square (43) Date of publication of application: London WC1A 2RA (GB) 24.06.1998 Bulletin 1998/26 (56) References cited: (73) Proprietor: Ortho-Clinical Diagnostics, Inc. EP-A- 0 421 788 EP-A- 0 603 954 Rochester, NY 14626-5101 (US) • INORG. CHIM. ACTA, vol. 243, no. 1-2, 1996, (72) Inventors: pages 201-206, XP000613696 J. V. WALKER ET • FRIEDMAN, Alan, Eric AL.: "Vanadium bromoperoxidase-catalyzed Rochester, NY 14617 (US) oxidation of thiocyanate by hydrogen peroxide." • KISSEL, Thomas, Robert Rochester, NY 14612 (US) Note: Within nine months from the publication of the mention of the grant of the European patent, any person may give notice to the European Patent Office of opposition to the European patent granted. -

Vanadium Haloperoxidases from Brown Algae of the Laminariaceae Family
Phytochemistry 57 (2001) 633–642 www.elsevier.com/locate/phytochem Vanadium haloperoxidases from brown algae of the Laminariaceae family M. Almeidaa, S. Filipea, M. Humanesa, M.F. Maiaa, R. Melob, N. Severinoa, J.A.L. da Silvac, J.J.R. Frau´ sto da Silvac,*, R. Weverd aCentro de Electroquı´mica e Cine´tica, Departamento de Quı´mica e Bioquı´mica, Faculdade de Cieˆncias da Universidade de Lisboa, Edifı´cio C1-5 piso, Campo Grande, 1749-016 Lisbon, Portugal bInstituto de Oceanografia, Faculdade de Cieˆncias da Universidade de Lisboa, Campo Grande, 1749-016 Lisbon, Portugal cCentro de Quı´mica Estrutural, Complexo 1, Instituto Superior Te´cnico, Av. Rovisco Pais,1, 1049-001 Lisbon, Portugal dInstitute for Molecular Chemistry, University of Amsterdam, Nieuwe Achtergracht 129, 1018 WS, Amsterdam, The Netherlands Received 25 July 2000; received in revised form 21 February 2001 Abstract Vanadium haloperoxidases were extracted, purified and characterized from three different species of Laminariaceae — Laminaria saccharina (Linne´ ) Lamouroux, Laminaria hyperborea (Gunner) Foslie and Laminaria ochroleuca de la Pylaie. Two different forms of the vanadium haloperoxidases were purified from L. saccharina and L. hyperborea and one form from L. ochroleuca species. Reconstitution experiments in the presence of several metal ions showed that only vanadium(V) completely restored the enzymes activity. The stability of some enzymes in mixtures of buffer solution and several organic solvents such as acetone, ethanol, methanol and 1-propanol was noteworthy; for instance, after 30 days at least 40% of the initial activity for some isoforms remained in mixtures of 3:1 buffer solution/organic solvent. The enzymes were also moderately thermostable, keeping full activity up to 40C. -

Polybrominated Dibenzo-P-Dioxins – Natural Formation Mechanisms and Biota Retention, Maternal Transfer, and Effects
Polybrominated dibenzo-p-dioxins – Natural formation mechanisms and biota retention, maternal transfer, and effects Kristina Arnoldsson Department of Chemistry Doctoral Thesis Umeå 2012 Kristina Arnnoldsson received her B.Sc. in Chemistry from Stockholm University. She began her graduate studies in Environmental Chemistry at the Department of Chemistry, Umeå University in 2007. Polybrominated dibenzo-p-dioxins (PBDD) and dibenzofurans (PBDF) are a group of compounds of emerging interest as potential environmental stressors. Their structures as well as toxic responses are similar to the highly characterized toxicants polychlorinated dibenzo-p-dioxins. High levels of PBDDs have been found in algae, shellfish and fish, even from remote areas in the Baltic Sea. The geographical and temporal variations of PBDD in biota samples suggests natural rather than anthropogenic origins. The work underlying this thesis investigated the retention and transfer behavior, as well as health and reproductive effects, of PBDD/Fs in fish, to further increase the knowledge on persistency, retention and effects of PBDD/Fs, specifically concerning influence of substitution pattern and physico-chemical properties. In addition, biotic and abiotic formation of PBDDs from naturally abundant phenolic precursors were explored, to evaluate whether the PBDD profiles found in Baltic Sea biota can be explained by natural formation processes. Department of Chemistry ISBN 978-91-7459-353-2 Umeå University, S-901 87 Umeå www.chemistry.umu.se Polybrominated dibenzo-p-dioxins – Natural formation mechanisms and biota retention, maternal transfer, and effects Kristina Arnoldsson Akademisk avhandling som med vederbörligt tillstånd av Rektor vid Umeå universitet för avläggande av filosofie doktorsexamen framläggs till offentligt försvar i hörsal KB3B1, KBC-huset, fredagen den 3 februari, kl.