2021 California Advantage Large Group 3-Tier HMO and PPO Prescription Drug List
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

DRUGS REQUIRING PRIOR AUTHORIZATION in the MEDICAL BENEFIT Page 1
Effective Date: 08/01/2021 DRUGS REQUIRING PRIOR AUTHORIZATION IN THE MEDICAL BENEFIT Page 1 Therapeutic Category Drug Class Trade Name Generic Name HCPCS Procedure Code HCPCS Procedure Code Description Anti-infectives Antiretrovirals, HIV CABENUVA cabotegravir-rilpivirine C9077 Injection, cabotegravir and rilpivirine, 2mg/3mg Antithrombotic Agents von Willebrand Factor-Directed Antibody CABLIVI caplacizumab-yhdp C9047 Injection, caplacizumab-yhdp, 1 mg Cardiology Antilipemic EVKEEZA evinacumab-dgnb C9079 Injection, evinacumab-dgnb, 5 mg Cardiology Hemostatic Agent BERINERT c1 esterase J0597 Injection, C1 esterase inhibitor (human), Berinert, 10 units Cardiology Hemostatic Agent CINRYZE c1 esterase J0598 Injection, C1 esterase inhibitor (human), Cinryze, 10 units Cardiology Hemostatic Agent FIRAZYR icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent HAEGARDA c1 esterase J0599 Injection, C1 esterase inhibitor (human), (Haegarda), 10 units Cardiology Hemostatic Agent ICATIBANT (generic) icatibant J1744 Injection, icatibant, 1 mg Cardiology Hemostatic Agent KALBITOR ecallantide J1290 Injection, ecallantide, 1 mg Cardiology Hemostatic Agent RUCONEST c1 esterase J0596 Injection, C1 esterase inhibitor (recombinant), Ruconest, 10 units Injection, lanadelumab-flyo, 1 mg (code may be used for Medicare when drug administered under Cardiology Hemostatic Agent TAKHZYRO lanadelumab-flyo J0593 direct supervision of a physician, not for use when drug is self-administered) Cardiology Pulmonary Arterial Hypertension EPOPROSTENOL (generic) -
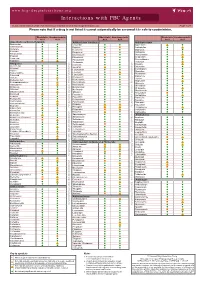
Interactions with PBC Agents
www.hep-druginteractions.org Interactions with PBC Agents Charts created March 2020. Full information available at www.hep-druginteractions.org Page 1 of 4 Please note that if a drug is not listed it cannot automatically be assumed it is safe to coadminister. Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Obeticholic Ursodeoxycholic Acid Acid Acid Acid Acid Acid Anaesthetics & Muscle Relaxants Antibacterials (continued) Antidepressants Bupivacaine Cloxacillin Agomelatine Cisatracurium Dapsone Amitriptyline Isoflurane Delamanid Bupropion Ketamine Ertapenem Citalopram Nitrous oxide Erythromycin Clomipramine Propofol Ethambutol Desipramine Thiopental Flucloxacillin Desvenlafaxine Tizanidine Gentamicin Dosulepin Analgesics Imipenem Doxepin Aceclofenac Isoniazid Duloxetine Alfentanil Escitalopram Aspirin Levofloxacin Linezolid Fluoxetine Buprenorphine Fluvoxamine Lymecycline distribution. Celecoxib Imipramine Meropenem Codeine Lithium Methenamine Dexketoprofen Maprotiline Metronidazole Dextropropoxyphene Mianserin Moxifloxacin Diamorphine Milnacipran Diclofenac Nitrofurantoin only. Not for distribution. for only. Not Mirtazapine Diflunisal Norfloxacin Moclobemide Dihydrocodeine Ofloxacin Nefazodone Etoricoxib Penicillin V Nortriptyline Fentanyl Piperacillin Paroxetine Flurbiprofen Pivmecillinam Sertraline Hydrocodone use ersonal Pyrazinamide Tianeptine Hydromorphone Rifabutin Trazodone Ibuprofen Rifampicin -

Does Your Patient Have Bile Acid Malabsorption?
NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 NUTRITION ISSUES IN GASTROENTEROLOGY, SERIES #198 Carol Rees Parrish, MS, RDN, Series Editor Does Your Patient Have Bile Acid Malabsorption? John K. DiBaise Bile acid malabsorption is a common but underrecognized cause of chronic watery diarrhea, resulting in an incorrect diagnosis in many patients and interfering and delaying proper treatment. In this review, the synthesis, enterohepatic circulation, and function of bile acids are briefly reviewed followed by a discussion of bile acid malabsorption. Diagnostic and treatment options are also provided. INTRODUCTION n 1967, diarrhea caused by bile acids was We will first describe bile acid synthesis and first recognized and described as cholerhetic enterohepatic circulation, followed by a discussion (‘promoting bile secretion by the liver’) of disorders causing bile acid malabsorption I 1 enteropathy. Despite more than 50 years since (BAM) including their diagnosis and treatment. the initial report, bile acid diarrhea remains an underrecognized and underappreciated cause of Bile Acid Synthesis chronic diarrhea. One report found that only 6% Bile acids are produced in the liver as end products of of British gastroenterologists investigate for bile cholesterol metabolism. Bile acid synthesis occurs acid malabsorption (BAM) as part of the first-line by two pathways: the classical (neutral) pathway testing in patients with chronic diarrhea, while 61% via microsomal cholesterol 7α-hydroxylase consider the diagnosis only in selected patients (CYP7A1), or the alternative (acidic) pathway via or not at all.2 As a consequence, many patients mitochondrial sterol 27-hydroxylase (CYP27A1). are diagnosed with other causes of diarrhea or The classical pathway, which is responsible for are considered to have irritable bowel syndrome 90-95% of bile acid synthesis in humans, begins (IBS) or functional diarrhea by exclusion, thereby with 7α-hydroxylation of cholesterol catalyzed interfering with and delaying proper treatment. -

PDCO Monthly Report of Opinions on Paediatric Investigation Plans and Other Activities 13-16 October 2020
16 October 2020 EMA/578758/2020 Human Medicines Division PDCO monthly report of opinions on paediatric investigation plans and other activities 13-16 October 2020 Opinions on paediatric investigation plans The Paediatric Committee (PDCO) adopted opinions agreeing paediatric investigation plans (PIPs) for the following medicines: • Bimekizumab, EMEA-002189-PIP03-19, from UCB Biopharma SRL, for the treatment of chronic idiopathic arthritis (including rheumatoid arthritis, spondyloarthritis, psoriatic arthritis and juvenile idiopathic arthritis); • Adrenaline (epinephrine), EMEA-002749-PIP01-19, from ARS Pharmaceuticals IRL, Limited, for the treatment of allergic reactions; • Guselkumab, EMEA-001523-PIP04-19, from Janssen-Cilag International N.V., for the treatment of ulcerative colitis; • Dapirolizumab pegol, EMEA-002702-PIP01-19, from UCB Biopharma SRL, for the treatment of systemic lupus erythematosus; • Allogeneic bone marrow derived mesenchymal stromal cells, ex-vivo expanded (MC0518), EMEA- 002706-PIP01-19, from medac Gesellschaft für klinische Spezialpräparate mbH, for the treatment of acute graft-versus-host disease; • Alpha1-proteinase inhibitor (human), EMEA-001312-PIP03-19, from CSL Behring GmbH, for the treatment of acute graft-versus-host disease; • Adeno-associated viral vector serotype 9 containing the human mini-dystrophin gene (PF- 06939926), EMEA-002741-PIP01-20, from Pfizer Europe MA EEIG, for the treatment of Duchenne Muscular Dystrophy; • Venglustat, EMEA-001716-PIP04-19, from Genzyme Europe B.V., for the treatment of -

(12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu Et Al
USOO9498431B2 (12) United States Patent (10) Patent No.: US 9.498,431 B2 Xu et al. (45) Date of Patent: Nov. 22, 2016 (54) CONTROLLED RELEASING COMPOSITION 7,053,134 B2 * 5/2006 Baldwin et al. .............. 522,154 2004/0058056 A1 3/2004 Osaki et al. ................... 427.2.1 (76) Inventors: Jianjian Xu, Hefei (CN); Shiliang 2005/0037047 A1 2/2005 Song Wang, Hefei (CN); Manzhi Ding 2007/0055364 A1* 3/2007 Hossainy .................. A61F 2/82 s: s s 623, 1.38 Hefei (CN) 2008/0274194 A1* 11/2008 Miller .................... A61K 9.146 424/489 (*) Notice: Subject to any disclaimer, the term of this patent is extended or adjusted under 35 FOREIGN PATENT DOCUMENTS U.S.C. 154(b) by 0 days. CN 1208.610 A 2, 1999 (21) Appl. No.: 13/133,656 EP O251680 A2 1, 1988 JP S63-22516. A 1, 1988 JP H1O-310518 A 11, 1998 (22) PCT Filed: Dec. 10, 2009 WO 96,10395 A1 4f1996 WO WO 2005.000277 A1 * 1, 2005 (86). PCT No.: PCT/CN2009/075468 WO 2007 115045 A2 10, 2007 WO 2008/OO2657 A2 1, 2008 S 371 (c)(1), WO 2008OO2657 A2 1, 2008 (2), (4) Date: Jun. 9, 2011 WO 2008041246 A2 4/2008 (87) PCT Pub. No.: WO2010/066203 OTHER PUBLICATIONS PCT Pub. Date: Jun. 17, 2010 Crowley and Zhang, Pharmaceutical Application of Hot Melt Extru (65) Prior Publication Data sion: Part I, Drug Development and Industrial Pharmacy, 2007. 33:909-926.* US 2011/024.4043 A1 Oct. 6, 2011 The Use of Poly (L-Lactide) and RGD Modified Microspheres as Cell Carriers in a Flow Intermittency Bioreactor for Tissue Engi (30) Foreign Application Priority Data neering Cartilage. -

Biomaterials: a Potential Pathway to Healing Chronic Wounds?
DR. BENJAMIN DAVID ALMQUIST (Orcid ID : 0000-0001-9718-777X) Received Date : 25-Feb-2016 Revised Date : 17-Nov-2016 Accepted Date : 28-Dec-2016 Article type : Viewpoint Biomaterials: A potential pathway to healing chronic wounds? Mara A Pop1 and Benjamin D Almquist1 1. Department of Bioengineering, Imperial College London, London SW7 2AZ, UK Article Corresponding Author: Benjamin D Almquist Email: [email protected] Declarations: None Key Words: biomaterials, chronic wound, wound healing, diabetic ulcer, tissue repair Abstract Chronic dermal wounds are a devastating problem that disproportionally affect individuals with conditions such as diabetes, paralysis, or simply old age. These wounds are extremely challenging to treat due to a heterogeneous combination of causative factors, creating a This article has been accepted for publication and undergone full peer review but has not been through the copyediting, typesetting, pagination and proofreading process, which may lead to differences between this version and the Version of Record. Please cite this article as Accepted doi: 10.1111/exd.13290 This article is protected by copyright. All rights reserved. substantial burden on healthcare systems worldwide. Despite their large impact, there is currently a startling lack of options for effectively treating the underlying biological changes that occur within the wounds. Biomaterials possess an enticing ability to provide new comprehensive approaches to healing these devastating wounds; advanced wound dressings are now being developed that enable the ability to coordinate temporal delivery of multiple therapeutics, protect sensitive biologics from degradation, and provide supportive matrices that encourage the growth of tissue. This positions biomaterials as a potential ‘conductor’ of wound repair, allowing them to simultaneously address numerous barriers to healing, and in turn providing a promising pathway to innovative new technologies for driving successful healing. -

Preferred Drug List 4-Tier
Preferred Drug List 4-Tier 21NVHPN13628 Four-Tier Base Drug Benefit Guide Introduction As a member of a health plan that includes outpatient prescription drug coverage, you have access to a wide range of effective and affordable medications. The health plan utilizes a Preferred Drug List (PDL) (also known as a drug formulary) as a tool to guide providers to prescribe clinically sound yet cost-effective drugs. This list was established to give you access to the prescription drugs you need at a reasonable cost. Your out- of-pocket prescription cost is lower when you use preferred medications. Please refer to your Prescription Drug Benefit Rider or Evidence of Coverage for specific pharmacy benefit information. The PDL is a list of FDA-approved generic and brand name medications recommended for use by your health plan. The list is developed and maintained by a Pharmacy and Therapeutics (P&T) Committee comprised of actively practicing primary care and specialty physicians, pharmacists and other healthcare professionals. Patient needs, scientific data, drug effectiveness, availability of drug alternatives currently on the PDL and cost are all considerations in selecting "preferred" medications. Due to the number of drugs on the market and the continuous introduction of new drugs, the PDL is a dynamic and routinely updated document screened regularly to ensure that it remains a clinically sound tool for our providers. Reading the Drug Benefit Guide Benefits for Covered Drugs obtained at a Designated Plan Pharmacy are payable according to the applicable benefit tiers described below, subject to your obtaining any required Prior Authorization or meeting any applicable Step Therapy requirement. -

Antimicrotubule Effects of Estramustine, an Antiprostatic Tumor Drug
[CANCER RESEARCH 45, 3891-3897, August 1985] Antimicrotubule Effects of Estramustine, an Antiprostatic Tumor Drug Mark E. Stearns1 and Kenneth D. Tew2 Departments of Anatomy [M. E. S.] and of Medicine and Pharmacology [K. D, T.], Vincent T. Lombardi Cancer Research Center, Georgetown University Hospital, Washington, DC 20007 ABSTRACT cytomatrix effects of EM and learn how cytoplasmic related effects of EM might ultimately produce the reported antimitotic Estramustine [170-estradiol 3 N bis(2-chloroethyl)carbamate; events in dividing cells (6). EM] is a stable conjugate of estradiol and nor-nitrogen mustard In this paper, we have investigated possible cytotoxic effects that is used for the treatment of human prostatic carcinoma. We of EM at the cytological level. For these studies, the fish erythro- have studied the cytotoxic effects of EM on the cytoskeletal phore or red pigment cell has been used as a model system for organization of squirrelfish pigment cells (erythrophores) and investigation of the cytotoxic consequences of EM. There are a human prostatic tumor cells (DU 145) in culture. Light and whole- number of attractive reasons for utilizing erythrophores for the mount electron microscopy studies reveal that, at /¿Mlevels(60 work described here. Erythrophores are symmetrical cells with to 120 ¿IM),EMhas a dose-dependent disruptive effect on cell thousands of radially ordered microtubules which control the shape, cytoskeletal organization, and intracellular transport. directed motion of numerous red pigment granules (14, 19). At Upon removal of the drug, the cytological effects of EM are the light microscopic level, the pigment is observed to pulsate or rapidly reversible in fish cells but not DU 145s. -

Tables-Of-Phase-3-Mabs.Pdf
Table 3. Monoclonal antibodies in Phase 2/3 or 3 clinical studies for cancer indications Most advanced Primary sponsoring company INN or code name Molecular format Target(s) phase Phase 3 indications Therapeutic area Janssen Research & Development, LLC JNJ-56022473 Humanized mAb CD123 Phase 2/3 Acute myeloid leukemia Cancer Murine IgG1, Actinium Pharmaceuticals Iomab-B radiolabeled CD45 Phase 3 Acute myeloid leukemia Cancer Humanized IgG1, Seattle Genetics Vadastuximab talirine ADC CD33 Phase 3 Acute myeloid leukemia Cancer TG Therapeutics Ublituximab Chimeric IgG1 CD20 Phase 3 Chronic lymphocytic leukemia Cancer Xencor XMAB-5574, MOR208 Humanized IgG1 CD19 Phase 2/3 Diffuse large B-cell lymphoma Cancer Moxetumomab Murine IgG1 dsFv, AstraZeneca/MedImmune LLC pasudotox immunotoxin CD22 Phase 3 Hairy cell leukemia Cancer Humanized scFv, Viventia Bio Oportuzumab monatox immunotoxin EpCAM Phase 3 Bladder cancer Cancer scFv-targeted liposome containing Merrimack Pharmaceuticals MM-302 doxorubicin HER2 Phase 2/3 Breast cancer Cancer MacroGenics Margetuximab Chimeric IgG1 HER2 Phase 3 Breast cancer Cancer Gastric cancer or gastroesophageal junction Gilead Sciences GS-5745 Humanized IgG4 MMP9 Phase 3 adenocarcinoma; ulcerative colitis (Phase 2/3) Cancer; Immune-mediated disorders Depatuxizumab Humanized IgG1, AbbVie mafodotin ADC EGFR Phase 2/3 Glioblastoma Cancer AstraZeneca/MedImmune LLC Tremelimumab Human IgG2 CTLA4 Phase 3 NSCLC, head & neck cancer, bladder cancer Cancer NSCLC, head & neck cancer, bladder cancer, breast AstraZeneca/MedImmune -

CDER Breakthrough Therapy Designation Approvals Data As of December 31, 2020 Total of 190 Approvals
CDER Breakthrough Therapy Designation Approvals Data as of December 31, 2020 Total of 190 Approvals Submission Application Type and Proprietary Approval Use Number Number Name Established Name Applicant Date Treatment of patients with previously BLA 125486 ORIGINAL-1 GAZYVA OBINUTUZUMAB GENENTECH INC 01-Nov-2013 untreated chronic lymphocytic leukemia in combination with chlorambucil Treatment of patients with mantle cell NDA 205552 ORIGINAL-1 IMBRUVICA IBRUTINIB PHARMACYCLICS LLC 13-Nov-2013 lymphoma (MCL) Treatment of chronic hepatitis C NDA 204671 ORIGINAL-1 SOVALDI SOFOSBUVIR GILEAD SCIENCES INC 06-Dec-2013 infection Treatment of cystic fibrosis patients age VERTEX PHARMACEUTICALS NDA 203188 SUPPLEMENT-4 KALYDECO IVACAFTOR 21-Feb-2014 6 years and older who have mutations INC in the CFTR gene Treatment of previously untreated NOVARTIS patients with chronic lymphocytic BLA 125326 SUPPLEMENT-60 ARZERRA OFATUMUMAB PHARMACEUTICALS 17-Apr-2014 leukemia (CLL) for whom fludarabine- CORPORATION based therapy is considered inappropriate Treatment of patients with anaplastic NOVARTIS lymphoma kinase (ALK)-positive NDA 205755 ORIGINAL-1 ZYKADIA CERITINIB 29-Apr-2014 PHARMACEUTICALS CORP metastatic non-small cell lung cancer (NSCLC) who have progressed on or are intolerant to crizotinib Treatment of relapsed chronic lymphocytic leukemia (CLL), in combination with rituximab, in patients NDA 206545 ORIGINAL-1 ZYDELIG IDELALISIB GILEAD SCIENCES INC 23-Jul-2014 for whom rituximab alone would be considered appropriate therapy due to other co-morbidities -

Immunfarmakológia Immunfarmakológia
Gergely: Immunfarmakológia Immunfarmakológia Prof Gergely Péter Az immunpatológiai betegségek döntő többsége gyulladásos, és ennek következtében általában szövetpusztulással járó betegség, melyben – jelenleg – a terápia alapvetően a gyulladás csökkentésére és/vagy megszűntetésére irányul. Vannak kizárólag gyulladásgátló gyógyszereink és vannak olyanok, amelyek az immunreakció(k) bénításával (=immunszuppresszió révén) vagy emellett vezetnek a gyulladás mérsékléséhez. Mind szerkezetileg, mind hatástanilag igen sokféle csoportba oszthatók, az alábbi felosztás elsősorban didaktikus célokat szolgál. 1. Nem-szteroid gyulladásgátlók (‘nonsteroidal antiinflammatory drugs’ NSAID) 2. Kortikoszteroidok 3. Allergia-elleni szerek (antiallergikumok) 4. Sejtoszlás-gátlók (citosztatikumok) 5. Nem citosztatikus hatású immunszuppresszív szerek 6. Egyéb gyulladásgátlók és immunmoduláns szerek 7. Biológiai terápia 1. Nem-szteroid gyulladásgátlók (NSAID) Ezeket a vegyületeket, melyek őse a szalicilsav (jelenleg, mint acetilszalicilsav ‘aszpirin’ használatos), igen kiterjedten alkalmazzák a reumatológiában, az onkológiában és az orvostudomány szinte minden ágában, ahol fájdalom- és lázcsillapításra van szükség. Egyes felmérések szerint a betegek egy ötöde szed valamilyen NSAID készítményt. Szerkezetük alapján a készítményeket több csoportba sorolhatjuk: szalicilátok (pl. acetilszalicilsav) pyrazolidinek (pl. fenilbutazon) ecetsav származékok (pl. indometacin) fenoxiecetsav származékok (pl. diclofenac, aceclofenac)) oxicamok (pl. piroxicam, meloxicam) propionsav -

Order in Council 1243/1995
PROVINCE OF BRITISH COLUMBIA ORDER OF THE LIEUTENANT GOVERNOR IN COUNCIL Order in Council No. 12 4 3 , Approved and Ordered OCT 121995 Lieutenant Governor Executive Council Chambers, Victoria On the recommendation of the undersigned, the Lieutenant Governor, by and with the advice and consent of the Executive Council, orders that Order in Council 1039 made August 17, 1995, is rescinded. 2. The Drug Schedules made by regulation of the Council of the College of Pharmacists of British Columbia, as set out in the attached resolution dated September 6, 1995, are hereby approved. (----, c" g/J1"----c- 4- Minister of Heal fandand Minister Responsible for Seniors Presidin Member of the Executive Council (This pan is for adnwustratlye purposes only and is not part of the Order) Authority under which Order Is made: Act and section:- Pharmacists, Pharmacy Operations and Drug Scheduling Act, section 59(2)(1), 62 Other (specify): - Uppodukoic1enact N6145; Resolution of the Council of the College of Pharmacists of British Columbia ("the Council"), made by teleconference at Vancouver, British Columbia, the 6th day of September 1995. RESOLVED THAT: In accordance with the authority established in Section 62 of the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia, S.B.C. Chapter 62, the Council makes the Drug Schedules by regulation as set out in the attached schedule, subject to the approval of the Lieutenant Governor in Council. Certified a true copy Linda J. Lytle, Phr.) Registrar DRUG SCHEDULES to the Pharmacists, Pharmacy Operations and Drug Scheduling Act of British Columbia The Drug Schedules have been printed in an alphabetical format to simplify the process of locating each individual drug entry and determining its status in British Columbia.