Epidemiological Measures of Risk of Malaria J
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Descriptive Statistics (Part 2): Interpreting Study Results
Statistical Notes II Descriptive statistics (Part 2): Interpreting study results A Cook and A Sheikh he previous paper in this series looked at ‘baseline’. Investigations of treatment effects can be descriptive statistics, showing how to use and made in similar fashion by comparisons of disease T interpret fundamental measures such as the probability in treated and untreated patients. mean and standard deviation. Here we continue with descriptive statistics, looking at measures more The relative risk (RR), also sometimes known as specific to medical research. We start by defining the risk ratio, compares the risk of exposed and risk and odds, the two basic measures of disease unexposed subjects, while the odds ratio (OR) probability. Then we show how the effect of a disease compares odds. A relative risk or odds ratio greater risk factor, or a treatment, can be measured using the than one indicates an exposure to be harmful, while relative risk or the odds ratio. Finally we discuss the a value less than one indicates a protective effect. ‘number needed to treat’, a measure derived from the RR = 1.2 means exposed people are 20% more likely relative risk, which has gained popularity because of to be diseased, RR = 1.4 means 40% more likely. its clinical usefulness. Examples from the literature OR = 1.2 means that the odds of disease is 20% higher are used to illustrate important concepts. in exposed people. RISK AND ODDS Among workers at factory two (‘exposed’ workers) The probability of an individual becoming diseased the risk is 13 / 116 = 0.11, compared to an ‘unexposed’ is the risk. -

Meta-Analysis of Proportions
NCSS Statistical Software NCSS.com Chapter 456 Meta-Analysis of Proportions Introduction This module performs a meta-analysis of a set of two-group, binary-event studies. These studies have a treatment group (arm) and a control group. The results of each study may be summarized as counts in a 2-by-2 table. The program provides a complete set of numeric reports and plots to allow the investigation and presentation of the studies. The plots include the forest plot, radial plot, and L’Abbe plot. Both fixed-, and random-, effects models are available for analysis. Meta-Analysis refers to methods for the systematic review of a set of individual studies with the aim to combine their results. Meta-analysis has become popular for a number of reasons: 1. The adoption of evidence-based medicine which requires that all reliable information is considered. 2. The desire to avoid narrative reviews which are often misleading. 3. The desire to interpret the large number of studies that may have been conducted about a specific treatment. 4. The desire to increase the statistical power of the results be combining many small-size studies. The goals of meta-analysis may be summarized as follows. A meta-analysis seeks to systematically review all pertinent evidence, provide quantitative summaries, integrate results across studies, and provide an overall interpretation of these studies. We have found many books and articles on meta-analysis. In this chapter, we briefly summarize the information in Sutton et al (2000) and Thompson (1998). Refer to those sources for more details about how to conduct a meta- analysis. -
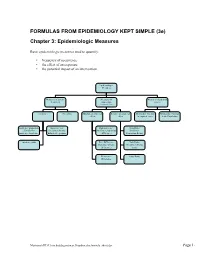
FORMULAS from EPIDEMIOLOGY KEPT SIMPLE (3E) Chapter 3: Epidemiologic Measures
FORMULAS FROM EPIDEMIOLOGY KEPT SIMPLE (3e) Chapter 3: Epidemiologic Measures Basic epidemiologic measures used to quantify: • frequency of occurrence • the effect of an exposure • the potential impact of an intervention. Epidemiologic Measures Measures of disease Measures of Measures of potential frequency association impact (“Measures of Effect”) Incidence Prevalence Absolute measures of Relative measures of Attributable Fraction Attributable Fraction effect effect in exposed cases in the Population Incidence proportion Incidence rate Risk difference Risk Ratio (Cumulative (incidence density, (Incidence proportion (Incidence Incidence, Incidence hazard rate, person- difference) Proportion Ratio) Risk) time rate) Incidence odds Rate Difference Rate Ratio (Incidence density (Incidence density difference) ratio) Prevalence Odds Ratio Difference Macintosh HD:Users:buddygerstman:Dropbox:eks:formula_sheet.doc Page 1 of 7 3.1 Measures of Disease Frequency No. of onsets Incidence Proportion = No. at risk at beginning of follow-up • Also called risk, average risk, and cumulative incidence. • Can be measured in cohorts (closed populations) only. • Requires follow-up of individuals. No. of onsets Incidence Rate = ∑person-time • Also called incidence density and average hazard. • When disease is rare (incidence proportion < 5%), incidence rate ≈ incidence proportion. • In cohorts (closed populations), it is best to sum individual person-time longitudinally. It can also be estimated as Σperson-time ≈ (average population size) × (duration of follow-up). Actuarial adjustments may be needed when the disease outcome is not rare. • In an open populations, Σperson-time ≈ (average population size) × (duration of follow-up). Examples of incidence rates in open populations include: births Crude birth rate (per m) = × m mid-year population size deaths Crude mortality rate (per m) = × m mid-year population size deaths < 1 year of age Infant mortality rate (per m) = × m live births No. -

CHAPTER 3 Comparing Disease Frequencies
© Smartboy10/DigitalVision Vectors/Getty Images CHAPTER 3 Comparing Disease Frequencies LEARNING OBJECTIVES By the end of this chapter the reader will be able to: ■ Organize disease frequency data into a two-by-two table. ■ Describe and calculate absolute and relative measures of comparison, including rate/risk difference, population rate/risk difference, attributable proportion among the exposed and the total population and rate/risk ratio. ■ Verbally interpret each absolute and relative measure of comparison. ■ Describe the purpose of standardization and calculate directly standardized rates. ▸ Introduction Measures of disease frequency are the building blocks epidemiologists use to assess the effects of a disease on a population. Comparing measures of disease frequency organizes these building blocks in a meaningful way that allows one to describe the relationship between a characteristic and a disease and to assess the public health effect of the exposure. Disease frequencies can be compared between different populations or between subgroups within a population. For example, one might be interested in comparing disease frequencies between residents of France and the United States or between subgroups within the U.S. population according to demographic characteristics, such as race, gender, or socio- economic status; personal habits, such as alcoholic beverage consump- tion or cigarette smoking; and environmental factors, such as the level of air pollution. For example, one might compare incidence rates of coronary heart disease between residents of France and those of the United States or 57 58 Chapter 3 Comparing Disease Frequencies among U.S. men and women, Blacks and Whites, alcohol drinkers and nondrinkers, and areas with high and low pollution levels. -

MMWR Early and Were Reported to Two Respective Local Health Jurisdictions, Release on the MMWR Website (
Morbidity and Mortality Weekly Report High SARS-CoV-2 Attack Rate Following Exposure at a Choir Practice — Skagit County, Washington, March 2020 Lea Hamner, MPH1; Polly Dubbel, MPH1; Ian Capron1; Andy Ross, MPH1; Amber Jordan, MPH1; Jaxon Lee, MPH1; Joanne Lynn1; Amelia Ball1; Simranjit Narwal, MSc1; Sam Russell1; Dale Patrick1; Howard Leibrand, MD1 On May 12, 2020, this report was posted as an MMWR Early and were reported to two respective local health jurisdictions, Release on the MMWR website (https://www.cdc.gov/mmwr). without indication of a common source of exposure. On On March 17, 2020, a member of a Skagit County, March 17, the choir director sent a second e-mail stating that Washington, choir informed Skagit County Public Health 24 members reported that they had developed influenza-like (SCPH) that several members of the 122-member choir had symptoms since March 11, and at least one had received test become ill. Three persons, two from Skagit County and one results positive for SARS-CoV-2. The email emphasized the from another area, had test results positive for SARS-CoV-2, importance of social distancing and awareness of symptoms the virus that causes coronavirus disease 2019 (COVID-19). suggestive of COVID-19. These two emails led many members Another 25 persons had compatible symptoms. SCPH to self-isolate or quarantine before a delegated member of the obtained the choir’s member list and began an investigation on choir notified SCPH on March 17. March 18. Among 61 persons who attended a March 10 choir All 122 members were interviewed by telephone either practice at which one person was known to be symptomatic, during initial investigation of the cluster (March 18–20; 53 cases were identified, including 33 confirmed and 20 115 members) or a follow-up interview (April 7–10; 117); most probable cases (secondary attack rates of 53.3% among con- persons participated in both interviews. -

Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple As It Can Get Chittaranjan Andrade, MD
Understanding Relative Risk, Odds Ratio, and Related Terms: As Simple as It Can Get Chittaranjan Andrade, MD Each month in his online Introduction column, Dr Andrade Many research papers present findings as odds ratios (ORs) and considers theoretical and relative risks (RRs) as measures of effect size for categorical outcomes. practical ideas in clinical Whereas these and related terms have been well explained in many psychopharmacology articles,1–5 this article presents a version, with examples, that is meant with a view to update the knowledge and skills to be both simple and practical. Readers may note that the explanations of medical practitioners and examples provided apply mostly to randomized controlled trials who treat patients with (RCTs), cohort studies, and case-control studies. Nevertheless, similar psychiatric conditions. principles operate when these concepts are applied in epidemiologic Department of Psychopharmacology, National Institute research. Whereas the terms may be applied slightly differently in of Mental Health and Neurosciences, Bangalore, India different explanatory texts, the general principles are the same. ([email protected]). ABSTRACT Clinical Situation Risk, and related measures of effect size (for Consider a hypothetical RCT in which 76 depressed patients were categorical outcomes) such as relative risks and randomly assigned to receive either venlafaxine (n = 40) or placebo odds ratios, are frequently presented in research (n = 36) for 8 weeks. During the trial, new-onset sexual dysfunction articles. Not all readers know how these statistics was identified in 8 patients treated with venlafaxine and in 3 patients are derived and interpreted, nor are all readers treated with placebo. These results are presented in Table 1. -

Outcome Reporting Bias in COVID-19 Mrna Vaccine Clinical Trials
medicina Perspective Outcome Reporting Bias in COVID-19 mRNA Vaccine Clinical Trials Ronald B. Brown School of Public Health and Health Systems, University of Waterloo, Waterloo, ON N2L3G1, Canada; [email protected] Abstract: Relative risk reduction and absolute risk reduction measures in the evaluation of clinical trial data are poorly understood by health professionals and the public. The absence of reported absolute risk reduction in COVID-19 vaccine clinical trials can lead to outcome reporting bias that affects the interpretation of vaccine efficacy. The present article uses clinical epidemiologic tools to critically appraise reports of efficacy in Pfzier/BioNTech and Moderna COVID-19 mRNA vaccine clinical trials. Based on data reported by the manufacturer for Pfzier/BioNTech vaccine BNT162b2, this critical appraisal shows: relative risk reduction, 95.1%; 95% CI, 90.0% to 97.6%; p = 0.016; absolute risk reduction, 0.7%; 95% CI, 0.59% to 0.83%; p < 0.000. For the Moderna vaccine mRNA-1273, the appraisal shows: relative risk reduction, 94.1%; 95% CI, 89.1% to 96.8%; p = 0.004; absolute risk reduction, 1.1%; 95% CI, 0.97% to 1.32%; p < 0.000. Unreported absolute risk reduction measures of 0.7% and 1.1% for the Pfzier/BioNTech and Moderna vaccines, respectively, are very much lower than the reported relative risk reduction measures. Reporting absolute risk reduction measures is essential to prevent outcome reporting bias in evaluation of COVID-19 vaccine efficacy. Keywords: mRNA vaccine; COVID-19 vaccine; vaccine efficacy; relative risk reduction; absolute risk reduction; number needed to vaccinate; outcome reporting bias; clinical epidemiology; critical appraisal; evidence-based medicine Citation: Brown, R.B. -

Copyright 2009, the Johns Hopkins University and John Mcgready. All Rights Reserved
This work is licensed under a Creative Commons Attribution-NonCommercial-ShareAlike License. Your use of this material constitutes acceptance of that license and the conditions of use of materials on this site. Copyright 2009, The Johns Hopkins University and John McGready. All rights reserved. Use of these materials permitted only in accordance with license rights granted. Materials provided “AS IS”; no representations or warranties provided. User assumes all responsibility for use, and all liability related thereto, and must independently review all materials for accuracy and efficacy. May contain materials owned by others. User is responsible for obtaining permissions for use from third parties as needed. Section F Measures of Association: Risk Difference, Relative Risk, and the Odds Ratio Risk Difference Risk difference (attributable risk)—difference in proportions - Sample (estimated) risk difference - Example: the difference in risk of HIV for children born to HIV+ mothers taking AZT relative to HIV+ mothers taking placebo 3 Risk Difference From csi command, with 95% CI 4 Risk Difference Interpretation, sample estimate - If AZT was given to 1,000 HIV infected pregnant women, this would reduce the number of HIV positive infants by 150 (relative to the number of HIV positive infants born to 1,000 women not treated with AZT) Interpretation 95% CI - Study results suggest that the reduction in HIV positive births from 1,000 HIV positive pregnant women treated with AZT could range from 75 to 220 fewer than the number occurring if the -

Epidemiology of and Risk Factors for COVID-19 Infection Among Health Care Workers: a Multi-Centre Comparative Study
International Journal of Environmental Research and Public Health Article Epidemiology of and Risk Factors for COVID-19 Infection among Health Care Workers: A Multi-Centre Comparative Study Jia-Te Wei 1, Zhi-Dong Liu 1, Zheng-Wei Fan 2, Lin Zhao 1,* and Wu-Chun Cao 1,2,* 1 Institute of EcoHealth, School of Public Health, Cheeloo College of Medicine, Shandong University, Jinan 250012, Shandong, China; [email protected] (J.-T.W.); [email protected] (Z.-D.L.) 2 State Key Laboratory of Pathogen and Biosecurity, Beijing Institute of Microbiology and Epidemiology, Beijing 100071, China; [email protected] * Correspondence: [email protected] (L.Z.); [email protected] (W.-C.C.) Received: 31 August 2020; Accepted: 28 September 2020; Published: 29 September 2020 Abstract: Healthcare workers (HCWs) worldwide are putting themselves at high risks of coronavirus disease 2019 (COVID-19) by treating a large number of patients while lacking protective equipment. We aim to provide a scientific basis for preventing and controlling the COVID-19 infection among HCWs. We used data on COVID-19 cases in the city of Wuhan to compare epidemiological characteristics between HCWs and non-HCWs and explored the risk factors for infection and deterioration among HCWs based on hospital settings. The attack rate (AR) of HCWs in the hospital can reach up to 11.9% in Wuhan. The time interval from symptom onset to diagnosis in HCWs and non-HCWs dropped rapidly over time. From mid-January, the median time interval of HCW cases was significantly shorter than in non-HCW cases. -

Measures of Effect & Potential Impact
Measures of Effect & Potential Impact Madhukar Pai, MD, PhD Assistant Professor McGill University, Montreal, Canada [email protected] 1 Big Picture Measures of Measures of Measures of potential disease freq effect impact First, we calculate measure of disease frequency in Group 1 vs. Group 2 (e.g. exposed vs. unexposed; treatment vs. placebo, etc.) Then we calculate measures of effect (which aims to quantify the strength of the association): Either as a ratio or as a difference: Ratio = (Measure of disease, group 1) / (Measure of disease, group 2) Difference = (Measure of disease, group 1) - (Measure of disease, group 2) Lastly, we calculate measures of impact, to address the question: if we removed or reduced the exposure, then how much of the disease burden can we reduce? Impact of exposure removal in the exposed group (AR, AR%) Impact of exposure remove in the entire population (PAR, PAR%) 2 Note: there is some overlap between measures of effect and impact 3 Measures of effect: standard 2 x 2 contingency epi table for count data (cohort or case-control or RCT or cross-sectional) Disease - Disease - no Column yes total (Margins) Exposure - a b a+b yes Exposure - c d c+d no Row total a+c b+d a+b+c+d (Margins) 4 Measures of effect: 2 x 2 contingency table for a cohort study or RCT with person-time data Disease - Disease - Total yes no Exposure - a - PYe yes Exposure – c - PY0 no Total a+c - PYe + PY0 Person-time in the unexposed group = PY0 5 Person-time in the exposed group = PYe STATA format for a 2x2 table [note that exposure -

Attributable Risk Estimation Using Sas/Stat Software
ATTRIBUTABLE RISK ESTIMATION USING SAS/STAT SOFTWARE Olaf Gefeller Abteilung Medizinische Statistik, Georg-August-Universitat Gottingen Abstract In epidemiologic studies devot~d to .the analysis of the relationship between an exposure characteristic and a disease the attributable risk constitutes an interesting epidemiologic risk measure. It can be interpreted as the proportion of cases of disease due to the exposure under study among all cases of disease in the study population. Thus, it indicates the impact of the exposure factor on the disease load of the population and can aid public health administrators in determining promising targets for disease preventive strategies in the population. The estimation of attributable risk from data arranged in contingency tables has received much attention in the statistical literature during recent years. However, none of the leading statistical software packages including SAS has incorporated the attributable risk in the catalogue of measures of association to be automatically calculated in some procedure. In the paper a brief introduction to the theory and application of attributable risk estimation from epidemiologic data is provided. Then, a general method is shown how to calculate attributable risk estimators under the multinomial sampling model usingSAS/STAT* software. Data from the Luebeck Blood Pressure Study, a cross-sectional study in the field of cardiovascular disease epidemiology, are used to illustrate the practical application of the method. Keywords: attributable risk, epidemiologic methods, measure of association 1. Introduction The quantification and measurement of disease risk associated with some exposure characteristic constitute a major goal of epidemiologic research. However, different conceptual approaches to describe the association between exposure and disease have been put forward in the ·epidemiologic literature. -
![Arxiv:1705.01135V2 [Q-Bio.QM] 17 Jul 2020 Author Summary](https://docslib.b-cdn.net/cover/8356/arxiv-1705-01135v2-q-bio-qm-17-jul-2020-author-summary-1308356.webp)
Arxiv:1705.01135V2 [Q-Bio.QM] 17 Jul 2020 Author Summary
Estimating and interpreting secondary attack risk: Binomial considered harmful Yushuf Sharker1, Eben Kenah2, * 1 Division of Biometrics, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland, USA 2 Biostatistics Division, College of Public Health, The Ohio State University, Columbus, Ohio, USA * [email protected] Abstract The household secondary attack risk (SAR), often called the secondary attack rate or secondary infection risk, is the probability of infectious contact from an infectious household member A to a given household member B, where we define infectious contact to be a contact sufficient to infect B if he or she is susceptible. Estimation of the SAR is an important part of understanding and controlling the transmission of infectious diseases. In practice, it is most often estimated using binomial models such as logistic regression, which implicitly attribute all secondary infections in a household to the primary case. In the simplest case, the number of secondary infections in a household with m susceptibles and a single primary case is modeled as a binomial(m; p) random variable where p is the SAR. Although it has long been understood that transmission within households is not binomial, it is thought that multiple generations of transmission can be safely neglected when p is small. We use probability generating functions and simulations to show that this is a mistake. The proportion of susceptible household members infected can be substantially larger than the SAR even when p is small. As a result, binomial estimates of the SAR are biased upward and their confidence intervals have poor coverage probabilities even if adjusted for clustering.