Metallurgy of Steel an Introduction for Knife Making
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Bainite - from Nano to Macro Symposium on Science and Application of Bainite 1/2 June 2017 Dorint Hotel, Wiesbaden, Germany
Bainite - from nano to macro Symposium on science and application of Bainite 1/2 June 2017 Dorint Hotel, Wiesbaden, Germany Honoring Professor Sir Harshad K. D. H. Bhadeshia Proceedings Bainite – from nano to macro, Wiesbaden (Germany), 1/2 June 2017 Copyright © 2017 Arbeitsgemeinschaft Wärmebehandlung und Werkstofftechnik e. V. All rights reserved. No part of the contents of this publication may be reproduced or transmitted in any form by any means without the written permission of the publisher. Arbeitsgemeinschaft Wärmebehandlung und Werkstofftechnik e. V. Paul-Feller-Str. 1 28199 Bremen Germany www.awt-online.org image sources: bulk nanostructured steel (coloured micrograph) Source: Garcia-Mateo, Caballero, Bhadeshia; Dorint Hotels & Resorts; Harshad K. D. H. Bhadeshia; Wiesbaden Marketing GmbH. Bainite – from nano to macro, Wiesbaden (Germany), 1/2 June 2017 International Committee H. Altena (Austria) S. Denis (France) I. Felde (Hungary) B. Haase (Germany) S. Hock (UK) P. Jacquot (France) Ch. Keul (Germany) B. Kuntzmann (Switzerland) V. Leskovśek (Slovenia) K. Löser (Germany) S. Mackenzie (USA) D. Petta (Italy) H.-W. Raedt (Germany) R. Schneider (Austria) B. Smoljan (Croatia) P. Stolař (Czech Republic) G. Totten (USA) E. Troell (Sweden) B. Vandewiele (Belgium) H.-J. Wieland (Germany) Bainite – from nano to macro, Wiesbaden (Germany), 1/2 June 2017 Sponsors of Bainite – from nano to macro Bainite – from nano to macro, Wiesbaden (Germany), 1/2 June 2017 Foreword Bainite – from nano to macro Symposium on science and application of bainite honoring Prof. Sir Harshad K. D. H. Bhadeshia, Tata Professor for Metallurgy and Director of SKF University Technology Center at the University Cambridge, UK and Professor for Computational Metallurgy at Pohang University of Science and Technology, Korea. -
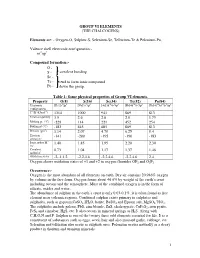
Group Vi Elements (The Chalcogens)
GROUP VI ELEMENTS (THE CHALCOGENS) Elements are: - Oxygen-O, Sulphur-S, Selenium-Se, Tellurium-Te & Polonium-Po. Valence shell electronic configuration:- ns2np4 Compound formation:- O - S - covalent bonding Se - Te - tend to form ionic compound Po - down the group. Table 1: Some physical properties of Group VI elements. Property O(8) S(16) Se(34) Te(52) Po(84) Electronic [He]2s22p4 [Ne]3s23p4 [Ar]3d104s24p4 [Kr]4d105s25p4 [Xe]4f145d106s26p4 configuration 1st IE (kJmol-1) 1314 1000 941 869 813 Electronegativity 3.5 2.6 2.6 2.0 1.75 Melting pt. (oC) -229 114 221 452 254 Boiling pt (oC) -183 445 685 869 813 Density (gm-3) 1.14 2.07 4.79 6.25 9.4 Electron -141 -200 -195 -190 -183 affinity,E- Ionic radius M2- 1.40 1.85 1.95 2.20 2.30 /Ao Covalent 0.73 1.04 1.17 1.37 1.46 radius/Ao Oxidation states -2,-1,1,2 -2,2,4,6 -2,2,4,6 -2,2,4,6 2,4 Oxygen shows oxidation states of +1 and +2 in oxygen fluorides OF2 and O2F2 Occurrence:- Oxygen is the most abundant of all elements on earth. Dry air contains 20.946% oxygen by volume in the free form. Oxygen forms about 46.6% by weight of the earth’s crust including oceans and the atmosphere. Most of the combined oxygen is in the form of silicate, oxides and water. The abundance of sulphur in the earth’s crust is only 0.03-0.1%. it is often found as free element near volcanic regions. -

High-Carbon Steels: Fully Pearlitic Microstructures and Applications
© 2005 ASM International. All Rights Reserved. www.asminternational.org Steels: Processing, Structure, and Performance (#05140G) CHAPTER 15 High-Carbon Steels: Fully Pearlitic Microstructures and Applications Introduction THE TRANSFORMATION OF AUSTENITE to pearlite has been de- scribed in Chapter 4, “Pearlite, Ferrite, and Cementite,” and Chapter 13, “Normalizing, Annealing, and Spheroidizing Treatments; Ferrite/Pearlite Microstructures in Medium-Carbon Steels,” which have shown that as microstructure becomes fully pearlitic as steel carbon content approaches the eutectiod composition, around 0.80% carbon, strength increases, but resistance to cleavage fracture decreases. This chapter describes the me- chanical properties and demanding applications for which steels with fully pearlitic microstructures are well suited. With increasing cooling rates in the pearlite continuous cooling trans- formation range, or with isothermal transformation temperatures ap- proaching the pearlite nose of isothermal transformation diagrams, Fig. 4.3 in Chapter 4, the interlamellar spacing of pearlitic ferrite and cementite becomes very fine. As a result, for most ferrite/pearlite microstructures, the interlamellar spacing is too fine to be resolved in the light microscope, and the pearlite appears uniformly dark. Therefore, to resolve the inter- lamellar spacing of pearlite, scanning electron microscopy, and for the finest spacings, transmission electron microscopy (TEM), are necessary to resolve the two-phase structure of pearlite. Figure 15.1 is a TEM mi- crograph showing very fine interlamellar structure in a colony of pearlite from a high-carbon steel rail. This remarkable composite structure of duc- © 2005 ASM International. All Rights Reserved. www.asminternational.org Steels: Processing, Structure, and Performance (#05140G) 282 / Steels: Processing, Structure, and Performance tile ferrite and high-strength cementite is the base microstructure for rail and the starting microstructure for high-strength wire applications. -

2000 Stainless Steels: an Introduction to Their Metallurgy and Corrosion
Dairy, Food and Environmental Sanitation, Vol. 20, No. 7, Pages 506-517 Copyright© International Association for Food Protection, 6200 Aurora Ave., Suite 200W, Des Moines, IA 50322 Stainless Steels: An Introduction to Their Metallurgy and Corrosion Resistance Roger A. Covert and Arthur H. Tuthill* and why they sometimes do not. In most cases, selection of the proper stainless steel leads to satisfactory performance. COMPOSITION, NOMEN- CLATURE AND GENERAL PROPERTIES Most metals are mixtures of a primary metallic element and one or more intentionally added other ele- This article has been peer-reviewed by two professionals. ments. These mixtures of elements are called alloys. Stainless steels are alloys, as are brasses (copper + zinc), bronzes (copper + tin), the many alu- INTRODUCTION better understanding of stainless minum alloys, and many other me- Worldwide, in industry, in busi- steels, especially to the non-metal- tallic materials. In general, solid ness and in the home, metals called lurgist. metals and alloys consist of randomly stainless steels are used daily. It is Industries are concerned with oriented grains that have a well-de- important to understand what these integrity of equipment and product fined crystalline structure, or lattice, materials are and why they behave purity. To achieve these, stainless within the grains. In stainless steels, the way they do. This is especially steels are often the economical and the crystalline structures within the true because the word “stainless” is practical materials of choice for pro- grains have been given names such as itself somewhat of a misnomer; these cess equipment. However, before ferrite, austenite, martensite, or a materials can stain and can corrode intelligent decisions can be made mixture of two or more of these. -

Carbon Steel
EN380 12-wk Exam Solution Fall 2019 Carbon Steel. 1. [19 pts] Three compositions of plain carbon steel are cooled very slowly in a turned-off furnace from ≈ 830◦C (see phase diagram below). For each composition, the FCC grains of γ−austenite (prior to transformation) are shown in an optical micrograph of the material surface. Sketch and label the phases making up the microstructures present in the right hand micrograph just after the austenite has completed transformation (note: the gray outlines of the prior γ grains may prove helpful). (a) [4 pts] C0 = 0:42% C (by wt). 830◦C 726◦C EN380 12-wk Exam Solution Page 1 Fall 2019 EN380 12-wk Exam Solution Fall 2019 (b) [4 pts] C0 = 0:80% C (by wt). 830◦C 726◦C (c) [4 pts] C0 = 1:05% C (by wt). 830◦C 726◦C (d) [7 pts] For the composition of part (c), C0 = 1:05% C (by wt), calculate the fraction of the solid that is pearlite at 726◦C. CF e3C − C0 6:67% − 1:05% Wpearlite = Wγ at 728◦C = = = 95:74% Pearlite CF e3C − Cγ 6:67% − 0:8% EN380 12-wk Exam Solution Page 2 Fall 2019 EN380 12-wk Exam Solution Fall 2019 2. [11 pts] Write in the correct term for each of the following related to carbon steels[1 pt each] (terms will be used exactly once): This material features carbon content in excess of Cast Iron 2:0% and is known for its excellent hardness, wear resistance, machinability and castability. -

Role of Austenitization Temperature on Structure Homogeneity and Transformation Kinetics in Austempered Ductile Iron
Metals and Materials International https://doi.org/10.1007/s12540-019-00245-y Role of Austenitization Temperature on Structure Homogeneity and Transformation Kinetics in Austempered Ductile Iron M. Górny1 · G. Angella2 · E. Tyrała1 · M. Kawalec1 · S. Paź1 · A. Kmita3 Received: 17 December 2018 / Accepted: 14 January 2019 © The Author(s) 2019 Abstract This paper considers the important factors of the production of high-strength ADI (Austempered Ductile Iron); namely, the austenitization stage during heat treatment. The two series of ADI with diferent initial microstructures were taken into consideration in this work. Experiments were carried out for castings with a 25-mm-walled thickness. Variable techniques (OM, SEM, dilatometry, DSC, Variable Magnetic Field, hardness, and impact strength measurements) were used for investi- gations of the infuence of austenitization time on austempering transformation kinetics and structure in austempered ductile iron. The outcome of this work indicates that the austenitizing temperature has a very signifcant impact on structure homo- geneity and the resultant mechanical properties. It has been shown that the homogeneity of the metallic matrix of the ADI microstructure strongly depends on the austenitizing temperature and the initial microstructure of the spheroidal cast irons (mainly through the number of graphite nodules). In addition, this work shows the role of the austenitization temperature on the formation of Mg–Cu precipitations in ADI. Keywords Metals · Casting · ADI · Heat treatment · Mg2Cu particles 1 Introduction light/heavy trucks, construction and mining equipment, railroad, agricultural, gears and crankshafts, and brackets, Austempered ductile iron (ADI) belongs to the spheroidal among others [5–7]. In the literature, numerous papers have graphite cast iron (SGI) family, which is subjected to heat been published on ADI: particularly, on the numerical sim- treatment; i.e., austenitization and austempering. -

Basic Keyword List
NOTICE TO AUTHORS 2008 Basic Keyword list A Antibodies Bismuth Chalcogens Ab initio calculations Antifungal agents Block copolymers Chaperone proteins Absorption Antigens Bond energy Charge carrier injection Acidity Antimony Bond theory Charge transfer Actinides Antisense agents Boranes Chelates Acylation Antitumor agents Borates Chemical vapor deposition Addition to alkenes Antiviral agents Boron Chemical vapor transport Addition to carbonyl com- Aqueous-phase catalysis Bridging ligands Chemisorption pounds Arene ligands Bromine Chemoenzymatic synthesis Adsorption Arenes Brønsted acids Chemoselectivity Aerobic oxidation Argon Chiral auxiliaries Aggregation Aromatic substitution C Chiral pool Agostic interactions Aromaticity C-C activation Chiral resolution Alanes Arsenic C-C bond formation Chirality Alcohols Arylation C-C coupling Chlorine Aldehydes Aryl halides C-Cl bond activation Chromates Aldol reaction Arynes C-Glycosides Chromium Alkali metals As ligands C-H activation Chromophores Alkaline earth metals Asymmetric amplification C1 building blocks Circular dichroism Alkaloids Asymmetric catalysis Cadmium Clathrates Alkanes Asymmetric synthesis Cage compounds Clays Alkene ligands Atmospheric chemistry Calcium Cleavage reactions Alkenes Atom economy Calixarenes Cluster compounds Alkylation Atropisomerism Calorimetry Cobalamines Alkyne ligands Aurophilicity Carbanions Cobalt Alkynes Autocatalysis Carbene homologues Cofactors Alkynylation Automerization Carbene ligands Colloids Allenes Autoxidation Carbenes Combinatorial chemistry Allosterism -

Preparation and Mechanical Behavior of Ultra-High Strength Low-Carbon Steel
materials Article Preparation and Mechanical Behavior of Ultra-High Strength Low-Carbon Steel Zhiqing Lv 1,2,*, Lihua Qian 1, Shuai Liu 1, Le Zhan 1 and Siji Qin 1 1 Key Laboratory of Advanced Forging & Stamping Technology and Science, Ministry of Education of China Yanshan University, Qinhuangdao 066004, China; [email protected] (L.Q.); [email protected] (S.L.); [email protected] (L.Z.); [email protected] (S.Q.) 2 State Key Laboratory of Metastable Material Science and Technology, Yanshan University, Qinhuangdao 066004, China * Correspondence: [email protected] Received: 16 December 2019; Accepted: 14 January 2020; Published: 18 January 2020 Abstract: The low-carbon steel (~0.12 wt%) with complete martensite structure, obtained by quenching, was cold rolled to get the high-strength steel sheets. Then, the mechanical properties of the sheets were measured at different angles to the rolling direction, and the microstructural evolution of low-carbon martensite with cold rolling reduction was observed. The results show that the hardness and the strength gradually increase with increasing rolling reduction, while the elongation and impact toughness obviously decrease. The strength of the sheets with the same rolling reduction are different at the angles of 0◦, 45◦, and 90◦ to the rolling direction. The tensile strength (elongation) along the rolling direction is higher than that in the other two directions, but the differences between them are not obvious. When the aging was performed at a low temperature, the strength of the initial martensite and deformed martensite increased with increasing aging time during the early stages of aging, followed by a gradual decrease with further aging. -

Chemical Analyses of Standard Sizes
SECTION P CPHEMICAL ANALYSES OF STANDARD SIZES STANDARD METALS AND DESIGNATION SYSTEMS . 2 EFFECTS OF COMMON ALLOYING ELEMENTS IN STEEL . 3-4 DESIGNATION OF CARBON STEELS . 5-7 DESIGNATION OF ALLOY STEELS .......................... 8-12 STAINLESS AND HEAT RESISTING STEELS .................. 13-17 HIGH TEMPERATURE HIGH STRENGTH ALLOYS . 18 DESIGNATION OF ALLUMINUM ALLOYS . 19-20 OIL TOOL MATERIALS . 21 API SPECIFICATION REQUIREMENTS ....................... 22 Sec. P Page 1 STANDARD METALS AND DESIGNATION SYSTEMS UNS Studies have been made in the metals industry for the purpose of establishing certain “standard” metals and eliminating as much as possible the manufacture of other metals which vary only slightly in composition from the standard metals. These standard metals are selected on the basis of serving the significant metal- lurgical and engineering needs of fabricators and users of metal products. UNIFIED NUMBERING SYSTEM: UNS is a system of designations established in accordance with ASTM E 527 and SAE J1086, Recommended Practice for Numbering Metals and Alloys. Its purpose is to provide a means of correlat- ing systems in use by such organizations as American Iron and Steel Institute (AISI), American Society for Testing Materials (ASTM), and Society of Automotive Engineers (SAE), as well as individual users and producers. UNS designa- tion assignments are processed by the SAE, the ASTM, or other relevant trade associations. Each of these assignors has the responsibility for administering a specific UNS series of designations. Each considers requests for the assignment of new UNS designations, and informs the applicants of the action taken. UNS designation assignors report immediately to the office of the Unified Numbering System for Metals and Alloys the details of each new assignment for inclusion into the system. -

Structure/Property Relationships in Irons and Steels Bruce L
Copyright © 1998 ASM International® Metals Handbook Desk Edition, Second Edition All rights reserved. J.R. Davis, Editor, p 153-173 www.asminternational.org Structure/Property Relationships in Irons and Steels Bruce L. Bramfitt, Homer Research Laboratories, Bethlehem Steel Corporation Basis of Material Selection ............................................... 153 Role of Microstructure .................................................. 155 Ferrite ............................................................. 156 Pearlite ............................................................ 158 Ferrite-Pearl ite ....................................................... 160 Bainite ............................................................ 162 Martensite .................................... ...................... 164 Austenite ........................................................... 169 Ferrite-Cementite ..................................................... 170 Ferrite-Martensite .................................................... 171 Ferrite-Austenite ..................................................... 171 Graphite ........................................................... 172 Cementite .......................................................... 172 This Section was adapted from Materials 5election and Design, Volume 20, ASM Handbook, 1997, pages 357-382. Additional information can also be found in the Sections on cast irons and steels which immediately follow in this Handbook and by consulting the index. THE PROPERTIES of irons and steels -

New Extremely Low Carbon Bainitic High-Strength Steel Bar Having Excellent Machinability and Toughness Produced by TPCP Technology*
KAWASAKI STEEL TECHNICAL REPORT No. 47 December 2002 New Extremely Low Carbon Bainitic High-Strength Steel Bar Having Excellent Machinability and Toughness Produced by TPCP Technology* Synopsis: A non heat-treated high strength steel bar for machine structural use through a thermo-mechanical precipita- tion control process (hereafter, referred to as TPCP) has been developed. The newly developed TPCP is a tech- nique for controlling the strength of the steel by precipi- tation hardening effected with the benefit of an extremely low carbon bainitic microstructure. The carbon content Kazukuni Hase Toshiyuki Hoshino Keniti Amano of the steel is decreased to below 0.02 mass% for realiz- Senior Researcher, Dr. Eng., Senior Dr. Eng., General Plate, Shape & Joining Researcher, Plate, Manager, Plate, Shape ing the proper microstructure, which improves both the Lab., Shape & Joining Lab., & Joining Lab., notch toughness and machinability. In order to make the Technical Res. Labs. Technical Res. Labs. Technical Res. Labs. microstructure bainitic and to obtain effective precipita- tion hardening, some micro-alloying elements are added. The developed steel manufactured with these advanced techniques showed a higher impact value, higher yield strength and better machinability than those 1 Introdution of the quenched and tempered AISI 4137 steel. The impact value of the steel is 250 J/cm2 or more at room In the fields of automobiles and industrial machines temperature. The problem of the reduction in yield ratio, where carbon steels and low alloy steels -

Black Carbon and Its Impact on Earth's Climate
Lesson Plan: Black Carbon and its Impact on Earth’s Climate A teacher-contributed lesson plan by Dr. Shefali Shukla, Sri Venkateswara College (University of Delhi), India. As a High School or Undergraduate Chemistry or Environmental Sciences teacher, you can use this set of computer-based tools to teach about allotropy, various allotropes of carbon and their structural and physical properties, black carbon, sources of black carbon and its impact on Earth’s climate. This lesson plan will help students understand the concept of allotropy and various allotropes of carbons. Students will learn about black carbon, the effect of black carbon on the Earth’s albedo and therefore, its impact on the climate. This lesson plan will also help students to understand how the immediate effect of controlling black carbon emission can potentially slow down the rate of global warming. Thus, the use of this lesson plan allows you to integrate the teaching of a climate science topic with a core topic in Chemistry or Environmental Sciences. Use this lesson plan to help your students find answers to: • What are allotropes? What are the various allotropes of carbon and their properties? • What are the sources of black carbon? • What are the different effects of black carbon on clouds? How does it modify rainfall pattern? • How does the deposition of black carbon on ice caps affect melting of the ice? • Explain how black carbon can have a cooling or warming effect on the planet? • What is the effect of black carbon on human health? About the Lesson Plan Grade Level: High School, Undergraduate Discipline: Chemistry, Environmental Sciences Topic(s) in Discipline: Allotropy, Allotropes of carbon, Black Carbon, Sources of Black Carbon, Heating and Cooling Effects of Black Carbon, Effect of Black Carbon on Human Health, Black Carbon Albedo, Black Carbon Emission Climate Topic: Climate and the Atmosphere, The Greenhouse Gas Effect, Climate and the Anthroposphere Location: Global Access: Online, Offline Language(s): English Approximate Time Required: 90-120 min 1 Contents 1.