Neurophysiological Assessment of Craniofacial Pain
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Blink Reflex, H-Reflex and Nerve-Conduction Alterations In
Lepr Rev (2006) 77, 114–120 Blink reflex, H-reflex and nerve-conduction alterations in leprosy patients ANA BERTHA MORA-BRAMBILA*, BENJAMI´N TRUJILLO-HERNA´ NDEZ**, RAFAEL COLL-CARDENAS***, MIGUEL HUERTA***, XO´ CHITL TRUJILLO***, CLEMENTE VA´ SQUEZ***, BERTHA ALICIA OLMEDO-BUENROSTRO*, REBECA O. MILLAN-GUERRERO** & ALEJANDRO ELIZALDE*** *Facultad de Enfermerı´a, Universidad de Colima, Colima, Me´xico **Unidad de Investigacio´n en Epidemiologı´a Clı´nica, Hospital General de Zona y Medicina Familiar No. 1, Instituto Mexicano del Seguro Social, Colima, Colima, Me´xico ***Centro Universitario de Investigaciones Biome´dicas, Universidad de Colima, Colima, Me´xico Accepted for publication 14 February 2006 Summary Peripheral nerve lesions are the most important cause of disability in leprosy patients. Electrophysiological studies are used in the diagnosis and prognosis of neuropathy. Nerve conduction is the most frequently used electrophysiological test method to detect neuropathy, although it evaluates only a part of the peripheral nervous system. Blink reflex and H-reflex are electrophysiological tests which evaluate facial and trigeminal nerve function. This study determined the frequencies of blink reflex, H-reflex and motor and sensory nerve conduction alterations in twenty five heterogeneous, clinic patients with lepromatous leprosy and a control group of 20 healthy subjects. Study results showed a decrease in motor and sensory nerve conduction in 40% and 30%, respectively. In blink reflex (BR), right R1 was altered in latency. in 20% of patients, left R1 in 20%, right ipsilateral R2 in 16%, left ipsilateral R2 in 20%, and right and left contralateral R2 were altered in 32% of patients. There was an absence of H-reflex in 16% (n ¼ 4) and prolonged latency in 4% (n ¼ 1). -

Territorial and Extraterritorial Trigeminocardiac Reflex: a Review for the Neurosurgeon and a Type IV Reflex Vignette
Open Access Review Article DOI: 10.7759/cureus.11646 Territorial and Extraterritorial Trigeminocardiac Reflex: A Review for the Neurosurgeon and a Type IV Reflex Vignette Daniel S. Leon-Ariza 1 , Juan S. Leon-Ariza 2 , Mayra A. Gualdron 3 , Jaime Bayona-Prieto 4 , Fidias E. Leon- Sarmiento 5, 6, 7 1. School of Medicine, Santander University-UDES, Bucaramanga, COL 2. Neuroscience, Mediciencias Research Group, Miami, USA 3. Faculty of Medicine, Unicolsanitas, Bogota, COL 4. Cirineo Research Group, Unicolciencias, Bucaramanga, COL 5. Environmental Health, Florida International University, Miami, USA 6. Neurology, Baptist Health South Florida, Miami Neuroscience Institute, Miami, USA 7. Internal Medicine, National University, Bogota, COL Corresponding author: Fidias E. Leon-Sarmiento, [email protected] Abstract The trigeminocardiac reflex (TCR) is a complex and, sometimes, fatal event triggered by overstimulation of the trigeminal nerve (TN) and its territorial and spinal cord branches. We reviewed and compiled for the neurosurgeon key aspects of the TCR that include a novel and straightforward classification, as well as morphophysiology, pathophysiology, neuromonitoring and neuromodulation features. Further, we present intraoperative data from a patient who developed extraterritorial, or type IV, TCR while undergoing a cervical surgery. TCR complexity, severity and unwanted outcomes indicate that this event should not be underestimated or overlooked in the surgical room. Timely TCR recognition in surgical settings is valuable for applying effective intraoperative management to prevent catastrophic outcomes. Categories: Otolaryngology, Neurosurgery, Anatomy Keywords: trigeminocardiac reflex trigeminal nerve, spinal cord, neurophysiology, neuromonitoring, neuromodulation Introduction And Background The trigeminocardiac reflex (TCR) is a complex neurovascular reflex triggered by overstimulating the trigeminal nerve (TN) and its anastomosis. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

The Neurological Exam
The Neurological Exam Introduction to the Neurological Exam The neurological exam consists of the following components: 1. Higher cognitive function as assessed by the mental status examination. (This will be addressed elsewhere in the course.) 2. Cranial nerves 3. Motor system 4. Sensory systems 5. Stance and gait I Olfactory Nerve Examination Technique: stimulant should be non-irritating test one nostril at a time with the opposite side occluded patient should not be able to see the stimulus cloves ideal stimulant since it preserves it’s scent improvise at bedside with soap, toothpaste, or perfume Normal Response: to perceive the scent with either nostril Abnormal Response: a unilateral loss is more likely to be significant and may imply a structural brain lesion affecting the olfactory bulb or tract, but could also be due to local causes such as a deviated septum or blocked nasal passage bilateral loss can occur with rhinitis or damage to the cribriform plate II Optic Nerve - Visual Acuity Examination Technique: each eye is tested separately. test best corrected vision using eyeglasses. any patient with uncorrected visual acuity of less than 20/20 should be examined with a pinhole. Improvement of vision through a pinhole indicates that the error is refractive. test distant vision using a Snellen chart at 10 or 20 feet. II Optic Nerve - Visual Fields A. Peripheral visual field (a) wiggling fingers (b) counting fingers (c) white pin B. Central visual field (a) red pin Examination Technique: visual fields are assessed by confrontation , i.e. the examiner compares the patient’s visual field to their own and assumes that theirs is normal. -

Brainstem Dysfunction in Critically Ill Patients
Benghanem et al. Critical Care (2020) 24:5 https://doi.org/10.1186/s13054-019-2718-9 REVIEW Open Access Brainstem dysfunction in critically ill patients Sarah Benghanem1,2 , Aurélien Mazeraud3,4, Eric Azabou5, Vibol Chhor6, Cassia Righy Shinotsuka7,8, Jan Claassen9, Benjamin Rohaut1,9,10† and Tarek Sharshar3,4*† Abstract The brainstem conveys sensory and motor inputs between the spinal cord and the brain, and contains nuclei of the cranial nerves. It controls the sleep-wake cycle and vital functions via the ascending reticular activating system and the autonomic nuclei, respectively. Brainstem dysfunction may lead to sensory and motor deficits, cranial nerve palsies, impairment of consciousness, dysautonomia, and respiratory failure. The brainstem is prone to various primary and secondary insults, resulting in acute or chronic dysfunction. Of particular importance for characterizing brainstem dysfunction and identifying the underlying etiology are a detailed clinical examination, MRI, neurophysiologic tests such as brainstem auditory evoked potentials, and an analysis of the cerebrospinal fluid. Detection of brainstem dysfunction is challenging but of utmost importance in comatose and deeply sedated patients both to guide therapy and to support outcome prediction. In the present review, we summarize the neuroanatomy, clinical syndromes, and diagnostic techniques of critical illness-associated brainstem dysfunction for the critical care setting. Keywords: Brainstem dysfunction, Brain injured patients, Intensive care unit, Sedation, Brainstem -

Lab Exercise 10
Lab Exercise 10 Sensory Tests Eye Anatomy Vision Tests Ear Anatomy Hearing Tests Textbook Reference: See Chapter 15 What you need to be able to do on the exam after completing this lab exercise: Be able to answer any question regarding the reflex/sensory tests, including the name of the reflex/sensory test, how it is performed, and what it is testing. For example, the patellar reflex test is testing the conduction of the femoral nerve. Be able to identify the listed parts of the eye on the eye models. Be able to answer any question regarding the vision tests, including the name of the test, how the test is performed, and what the results mean. Be able to identify the listed parts of the ear on the ear models. Be able to answer any question regarding the hearing tests, including the name of the test, how the test is performed, and what the results mean. 10-1 Sensory Tests Reflexes are involuntary, instantaneous movements in response to stimuli. Reflexes are mediated via a reflex arc, which includes a receptor, sensory neuron, integration center, motor neuron, and effector. Stretch Reflexes A stretch reflex is a muscle contraction in response to stretching within a muscle. Patellar Reflex The patellar (knee-jerk) reflex is an example of a stretch reflex. The patellar reflex tests the conduction of the femoral nerve. 1. Sit on the lab bench with your feet dangling down. 2. Have your lab partner tap the patellar ligament with the blunt side of a patellar reflex hammer. The tap should be 3-4 inches below the kneecap, and firm, but not hard enough to hurt. -

Diagnosis of Brain Death, Back to Medical Diagnosis!
Anaesth Crit Care Pain Med 38 (2019) 117–118 Editorial Diagnosis of brain death, back to medical diagnosis! ARTICLE INFO be different in real life. Similarly, the results are purely declarative and may therefore be different. Second, there are some things that can help Keywords: the doctor improve the way BD is diagnosed. In France, in most Absent brain stem reflexes hospitals, transplant coordinators (nurses or doctors) come to the ICU Apnoea test to help the team in the donation process. The impact of the transplant Brain death Brain death diagnosis Irreversible brain coordinator on the diagnosis of BD is not discussed. In addition, many injury ICU have developed procedures and checklists for the diagnosis of BD. It is important to know if they have the documents available. Thirdly, the criteria for identifying the "experienced doctor" are questionable. Experience, educational programs followed, number of donors Brain dead donors are the most common source of organs. The diagnosed are criteria that could be more relevant. diagnosis of brain death is a very important step in organ donation. Nevertheless, this work raises the issue of the diagnosis of BD. It ICU physicians must know how to make a correct diagnosis. seems that doctors do not diagnose BD in a correct way and as required The concept of brain death (BD) is different between countries. by law. The concept of "whole brain death" characterised by irreversible With regard to the different laws and recommendations cessation of hemispheric neurological functions and brain stem is between countries, it is important that the diagnosis of BD the most common. -

Intracranial Stimulation of the Trigeminal Nerve in Man II
Journal of Neurology, Neurosurgery, and Psychiatry 1986;49:419-427 Intracranial stimulation of the trigeminal nerve in man II. Reflex responses GIORGIO CRUCCU, DAVID BOWSHER From the Pain ReliefFoundation and Department ofNeurosciences, Walton Hospital, Liverpool, UK SUMMARY The reflex responses evoked by direct electrical stimulation of the intracranial portion of the trigeminal nerve have been studied in 16 subjects undergoing percutaneous retrogasserian ther- mocoagulation for the treatment of trigeminal neuralgia affecting the second or third division. In the obicularis oculi muscle, early and late responses similar to the RI and R2 components of the blink reflex were recorded. The former could be evoked only by stimulation of the second division and its latency was consistent with intermediately fast afferents. A late reflex (50-70 ms) was occa- sionally recorded from the anterior belly of the digastric muscle. The response was sometimes followed by a later activity and showed the features of a polysynaptic reflex. No response was obtained in the jaw elevators when fully relaxed. With the subject voluntarily clenching his teeth, both an early "H-like" response and two silent periods in the background EMG were obtained. The second silent period was similar in the muscles ipsi- and contralateral to intracranial stimulation, while the first silent period was longer in the ipsilateral muscles. Possible mechanisms contributing to the inhibition following stimulation of the mixed portion of the nerve are discussed. Owing to difficulties -
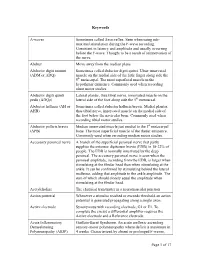
Of 17 Keywords A-Waves Sometimes Called Axon Reflex. Seen
Keywords A-waves Sometimes called Axon reflex. Seen when using sub- maximal stimulation during the F-wave recording. Consistent in latency and amplitude and usually occurring before the F-wave. Thought to be a result of reinnervation of the nerve. Abduct Move away from the median plane Abductor digiti minimi Sometimes called abductor digiti quinti. Ulnar innervated (ADM or ADQ) muscle on the medial side of the little finger along side the 5th metacarpal. The most superficial muscle in the hypothenar eminence. Commonly used when recording ulnar motor studies. Abductor digiti quinti Lateral plantar, thus tibial nerve, innervated muscle on the pedis (ADQp) lateral side of the foot along side the 5th metatarsal. Abductor hallucis (AH or Sometimes called abductor hallucis brevis. Medial plantar, AHB) thus tibial nerve, innervated muscle on the medial side of the foot below the navicular bone. Commonly used when recording tibial motor studies. Abductor pollicis brevis Median innervated muscle just medial to the 1st metacarpal (APB) bone. The most superficial muscle of the thenar eminence. Commonly used when recording median motor studies. Accessory peroneal nerve A branch of the superficial peroneal nerve that partly supplies the extensor digitorum brevis (EDB) in 18-22% of people. The EDB is normally innervated by the deep peroneal. The accessory peroneal nerve is seen when the peroneal amplitude, recording from the EDB, is larger when stimulating at the fibular head than when stimulating at the ankle. It can be confirmed by stimulating behind the lateral malleous, adding that amplitude to the ankle amplitude. The sum of which should closely equal the amplitude when stimulating at the fibular head. -

Loss of Brainstem Reflexes: Not Always an Ominous Sign
Netherlands Journal of Critical Care Submitted May 2018; Accepted September 2018 CASE REPORT Loss of brainstem reflexes: not always an ominous sign V.L. Winia1, J. Horn3, B. van den Bogaard2,3 1Department of Intensive Care, Netherlands Cancer Institute, Amsterdam, the Netherlands 2Department of Intensive Care, OLVG Oost, Amsterdam, the Netherlands 3Department of Intensive Care, Amsterdam UMC, location AMC, University of Amsterdam, Amsterdam, the Netherlands Correspondence V.L. Winia – [email protected] Keywords - brainstem reflexes, intoxication, brain death Abstract and oculocephalic reflexes were absent. Plantar reflexes were We present three cases of serious multidrug intoxications indifferent. CT of the brain did not show any abnormalities, with absent brainstem reflexes that may imply diagnostic and specifically no signs of cerebral oedema or elevated intracranial prognostic challenges. Given the good neurological outcome in pressure, and CT angiography of the brain excluded basilar these patients and of earlier described cases in the literature, the artery thrombosis. ECG on admission showed a right axis, absence of brainstem reflexes in intoxication is not necessarily prolonged PQ interval (144 ms) and a broad QRS complex (160 a sign of a poor prognosis and should be used with caution in ms). There was a strong suspicion of intentional intoxication prognostication. with amitriptyline and as such activated charcoal and sodium bicarbonate were administered. Amitriptyline serum levels Introduction were found to be seriously elevated (amitriptyline >730 µg/l, Absent brainstem reflexes in the presence of known brain injury nortriptyline 400 µg/l). On day 1 of her admission she had yet is often regarded as a sign of serious brainstem injury. -

Corneal Reflex in Hemisphere Disease
Journal ofNeurology, Neurosurgery, and Psychiatry, 1972, 35, 877-880 J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.35.6.877 on 1 December 1972. Downloaded from Corneal reflex in hemisphere disease R. T. ROSS From the Section ofNeurology, Department of Medicine, University of Manitoba, and the Winnipeg General Hospital, Winnipeg R3E OZ3, Canada SUMMARY The contralateral corneal reflex may be absent in patients with a deep lesion of the parietal lobe. Frontal and temporal lobe lesions apparently do not interfere with this reflex. The corneal reflex is part of the neurological paresis was stimulated. The increase, however, examination. In lesions of the fifth and seventh was only a few milliseconds and the number of cranial nerves, as well as intrinsic disease of the cases was thought to be too small for any valid brain-stem, the integrity of the corneal reflex conclusions. may contribute to the diagnosis. In other studies of the blink reflex by Rush- The purpose of this paper is to show the worth (1962), Bender (1968), Bender, Maynard, corneal reflex as a superficial reflex, somewhat and Hastings (1969), Kimura, Powers, and Van analogous to the abdominal reflexes, which may Allen (1969), and Young and Shahani (1969), guest. Protected by copyright. disappear in disease of the contralateral hemi- there are few, if any, references to hemisphere sphere. Oliver (1952) described three patients, lesions affecting the reflex on the appropriate each of whom had an intracerebral lesion and an side. abnormal corneal reflex. The first had a left The following are brief summaries of 13 intracerebral, frontoparietal haematoma with an patients, all with intracranial lesions and some absent right corneal reflex; the second a left with an absent corneal reflex on the appropriate frontoparietal tumour with an absent right side. -

Electrical Study Ofjaw and Orbicularis Oculi Reflexes After Trigeminal Nerve
J Neurol Neurosurg Psychiatry: first published as 10.1136/jnnp.41.9.819 on 1 September 1978. Downloaded from Journal ofNeurology, Neurosurgery, and Psychiatry, 1978, 41, 819-823 Electrical study of jaw and orbicularis oculi reflexes after trigeminal nerve surgery I. T. FERGUSON From the Department of Neurology, Royal Infirmary, Dundee S U M M A R Y Trigeminal nerve ophthalmic and motor division function was assessed clinically and electrically in 32 patients who had undergone various surgical procedures for trigeminal neuralgia. Using known electrophysiological techniques, the orbicularis oculi and jaw reflexes were tested in all subjects. Abnormalities of the orbicularis oculi reflex were anticipated on the basis of ophthalmic division anaesthesia. However, jaw reflex abnormalities appeared in operated cases with no clinical or electromyographic evidence of masseter denervation. These results were unexpected, and imply that the proprioceptive fibres of the jaw reflex are mediated by a sensory and not a motor root as previously believed. guest. Protected by copyright. Electromyographic techniques have been applied and Lyon, 1972; Shahani and Young, 1972). Early in the past to the study of lesions affecting the and late components share a common efferent path trigeminal nerve (Kimura et al., 1970; Goor and in the facial nerve. Ongerboer De Visser, 1976). Abnormalities have Experimental animal work has led to the belief been demonstrated in the orbicularis oculi (blink) that the jaw jerk in man is a monosynaptic stretch reflex and jaw (jerk) reflex in patients with early response with specialised brainstem connections cerebellopontine angle tumours, and demyelinat- whose afferent and efferent fibres share a common ing, and vascular lesions affecting the brainstem pathway in the motor root of the trigeminal nerve (Goodwill and O'Tauma, 1969; Kimura and Lyon, (Corbin and Harrison, 1940; Szentagothai, 1948; 1972; Lyon and Van Allen, 1972; Eisen and Danon, McIntyre, 1951; Jerge, 1963).