The Genus Ptychogaster
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

A Phylogenetic Overview of the Antrodia Clade (Basidiomycota, Polyporales)
Mycologia, 105(6), 2013, pp. 1391–1411. DOI: 10.3852/13-051 # 2013 by The Mycological Society of America, Lawrence, KS 66044-8897 A phylogenetic overview of the antrodia clade (Basidiomycota, Polyporales) Beatriz Ortiz-Santana1 phylogenetic studies also have recognized the genera Daniel L. Lindner Amylocystis, Dacryobolus, Melanoporia, Pycnoporellus, US Forest Service, Northern Research Station, Center for Sarcoporia and Wolfiporia as part of the antrodia clade Forest Mycology Research, One Gifford Pinchot Drive, (SY Kim and Jung 2000, 2001; Binder and Hibbett Madison, Wisconsin 53726 2002; Hibbett and Binder 2002; SY Kim et al. 2003; Otto Miettinen Binder et al. 2005), while the genera Antrodia, Botanical Museum, University of Helsinki, PO Box 7, Daedalea, Fomitopsis, Laetiporus and Sparassis have 00014, Helsinki, Finland received attention in regard to species delimitation (SY Kim et al. 2001, 2003; KM Kim et al. 2005, 2007; Alfredo Justo Desjardin et al. 2004; Wang et al. 2004; Wu et al. 2004; David S. Hibbett Dai et al. 2006; Blanco-Dios et al. 2006; Chiu 2007; Clark University, Biology Department, 950 Main Street, Worcester, Massachusetts 01610 Lindner and Banik 2008; Yu et al. 2010; Banik et al. 2010, 2012; Garcia-Sandoval et al. 2011; Lindner et al. 2011; Rajchenberg et al. 2011; Zhou and Wei 2012; Abstract: Phylogenetic relationships among mem- Bernicchia et al. 2012; Spirin et al. 2012, 2013). These bers of the antrodia clade were investigated with studies also established that some of the genera are molecular data from two nuclear ribosomal DNA not monophyletic and several modifications have regions, LSU and ITS. A total of 123 species been proposed: the segregation of Antrodia s.l. -

Polypore Diversity in North America with an Annotated Checklist
Mycol Progress (2016) 15:771–790 DOI 10.1007/s11557-016-1207-7 ORIGINAL ARTICLE Polypore diversity in North America with an annotated checklist Li-Wei Zhou1 & Karen K. Nakasone2 & Harold H. Burdsall Jr.2 & James Ginns3 & Josef Vlasák4 & Otto Miettinen5 & Viacheslav Spirin5 & Tuomo Niemelä 5 & Hai-Sheng Yuan1 & Shuang-Hui He6 & Bao-Kai Cui6 & Jia-Hui Xing6 & Yu-Cheng Dai6 Received: 20 May 2016 /Accepted: 9 June 2016 /Published online: 30 June 2016 # German Mycological Society and Springer-Verlag Berlin Heidelberg 2016 Abstract Profound changes to the taxonomy and classifica- 11 orders, while six other species from three genera have tion of polypores have occurred since the advent of molecular uncertain taxonomic position at the order level. Three orders, phylogenetics in the 1990s. The last major monograph of viz. Polyporales, Hymenochaetales and Russulales, accom- North American polypores was published by Gilbertson and modate most of polypore species (93.7 %) and genera Ryvarden in 1986–1987. In the intervening 30 years, new (88.8 %). We hope that this updated checklist will inspire species, new combinations, and new records of polypores future studies in the polypore mycota of North America and were reported from North America. As a result, an updated contribute to the diversity and systematics of polypores checklist of North American polypores is needed to reflect the worldwide. polypore diversity in there. We recognize 492 species of polypores from 146 genera in North America. Of these, 232 Keywords Basidiomycota . Phylogeny . Taxonomy . species are unchanged from Gilbertson and Ryvarden’smono- Wood-decaying fungus graph, and 175 species required name or authority changes. -

SOMA Speaker: Catharine Adams March 17 at the Sonoma County Farm Bureau “How the Death Cap Mushroom Conquered the World”
SOMANEWS From the Sonoma County Mycological Association VOLUME 28: 7 MARCH 2016 SOMA Speaker: Catharine Adams March 17 At the Sonoma County Farm Bureau “How the Death Cap Mushroom Conquered the World” Cat Adams is interested in how chemical ecology in- fluences interactions between plants and fungi. For her PhD in Tom Bruns’ lab, Cat is studying the inva- sive ectomycorrhizal fungus, Amanita phalloides. The death cap mushroom kills more people than any oth- er mushroom, but how the deadly amatoxins influ- ence its invasion remains unexplored. Previously, Cat earned her M.A. with Anne Pringle at Harvard University. Her thesis examined fungal pathogens of the wild Bolivian chili pepper, Capsi- cum chacoense, and how the fungi evolved tolerance to spice. With the Joint Genome Institute, she is now sequencing the genome of one fungal isolate, a Pho- mopsis species, to better understand the novel en- zymes these fungi wield to outwit their plant host. She also collaborates with a group in China, study- loides, was an invasive species, and why we should ing how arbuscular mycorrhizae can help crop plants care. She’ll then tell you about 10 years of research at avoid toxic effects from pollution. Their first paper is Pt Reyes National Seashore examining how Amanita published in Chemosphere. phalloides spreads. Lastly, Cat will outline her ongo- At the SOMA meeting, Cat will explain how scientists ing work to determine the ecological role of Phalloi- determined the death cap mushroom, Amanita phal- des’ toxins, and will present her preliminary findings. NEED EMERGENCY MUSHROOM POISONING ID? After seeking medical attention, contact Darvin DeShazer for identification at (707) 829- 0596. -

A Revised Family-Level Classification of the Polyporales (Basidiomycota)
fungal biology 121 (2017) 798e824 journal homepage: www.elsevier.com/locate/funbio A revised family-level classification of the Polyporales (Basidiomycota) Alfredo JUSTOa,*, Otto MIETTINENb, Dimitrios FLOUDASc, € Beatriz ORTIZ-SANTANAd, Elisabet SJOKVISTe, Daniel LINDNERd, d €b f Karen NAKASONE , Tuomo NIEMELA , Karl-Henrik LARSSON , Leif RYVARDENg, David S. HIBBETTa aDepartment of Biology, Clark University, 950 Main St, Worcester, 01610, MA, USA bBotanical Museum, University of Helsinki, PO Box 7, 00014, Helsinki, Finland cDepartment of Biology, Microbial Ecology Group, Lund University, Ecology Building, SE-223 62, Lund, Sweden dCenter for Forest Mycology Research, US Forest Service, Northern Research Station, One Gifford Pinchot Drive, Madison, 53726, WI, USA eScotland’s Rural College, Edinburgh Campus, King’s Buildings, West Mains Road, Edinburgh, EH9 3JG, UK fNatural History Museum, University of Oslo, PO Box 1172, Blindern, NO 0318, Oslo, Norway gInstitute of Biological Sciences, University of Oslo, PO Box 1066, Blindern, N-0316, Oslo, Norway article info abstract Article history: Polyporales is strongly supported as a clade of Agaricomycetes, but the lack of a consensus Received 21 April 2017 higher-level classification within the group is a barrier to further taxonomic revision. We Accepted 30 May 2017 amplified nrLSU, nrITS, and rpb1 genes across the Polyporales, with a special focus on the Available online 16 June 2017 latter. We combined the new sequences with molecular data generated during the Poly- Corresponding Editor: PEET project and performed Maximum Likelihood and Bayesian phylogenetic analyses. Ursula Peintner Analyses of our final 3-gene dataset (292 Polyporales taxa) provide a phylogenetic overview of the order that we translate here into a formal family-level classification. -

Buckinghamshire Fungus Group Newsletter No. 13 August 2012
BFG Buckinghamshire Fungus Group Newsletter No. 13 August 2012 £3.75 to non-members The BFG Newsletter is published annually in August or September by the Buckinghamshire Fungus Group. The group was established in 1998 with the aim of: encouraging and carrying out the recording of fungi in Buckinghamshire and elsewhere; encouraging those with an interest in fungi and assisting in expanding their knowledge; generally promoting the study and conservation of fungi and their habitats. Secretary and Joint Recorder Derek Schafer Newsletter Editor and Joint Recorder Penny Cullington Membership Secretary Toni Standing Programme Secretary Joanna Dodsworth Webmaster Peter Davis Database manager Nick Jarvis The Group can be contacted via our website www.bucksfungusgroup.org.uk , by email at [email protected] , or at the address on the back page of the Newsletter. Membership costs £4.50 a year for a single member, £6 a year for families, and members receive a free copy of this Newsletter. No special expertise is required for membership, all are welcome, particularly beginners. CONTENTS WELCOME! Penny Cullington 3 BITS AND BOBS " 3 FORAY PROGRAMME " 4 REPORT ON THE 2011 SEASON Derek Schafer 5 MORE ON THE ROCK ROSE AMANITA STORY Penny Cullington 18 SOME MORE NAME CHANGES " 19 HAVE YOU SEEN THIS FUNGUS? " 20 SOME INTERESTING BLACK DOTS ON STICKS " 20 WHEN IS A SLIME MOULD NOT A SLIME MOULD? " 22 ROMAN FUNGAL HISTORY Brian Murray 26 HERICIUM ERINACEUS ON NAPHILL COMMON Peter Davis 29 AN INDENTIFICATION CHALLENGE Penny Cullington 33 EXPLORING THE ORIGINS OF SOME LATIN NAMES " 34 AN INDENTIFICATION CHALLENGE – THE ANSWER " 39 Photo credits, all ©: PC = Penny Cullington; PD = Peter Davis; Justin Long = JL; DJS = Derek Schafer; NS = Nick Standing Cover photo: Lentinus tigrinus photographed beside the lake at the Wotton House Estate, 4 Sep 2011 (DJS) 2 WELCOME! Welcome all to our 2012 newsletter which we hope will fill you with enthusiasm for the coming foray season. -
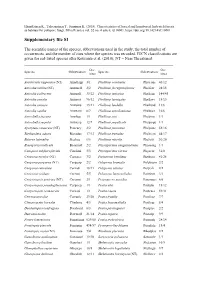
Supplementary File S1
Hämäläinen K., Tahvanainen T., Junninen K. (2018). Characteristics of boreal and hemiboreal herb-rich forests as habitats for polypore fungi. Silva Fennica vol. 52 no. 4 article id 10001. https://doi.org/10.14214/sf.10001 Supplementary file S1 The scientific names of the species, abbreviations used in the study, the total number of occurrences, and the number of sites where the species was recorded. IUCN classifications are given for red-listed species after Kotiranta et al. (2010); NT = Near Threatened. Occ. Occ. Species Abbreviation Species Abbreviation /sites /sites Amylocystis lapponica (NT) Amyllapp 3/1 Phellinus conchatus Phelconc 46/12 Antrodia mellita (NT) Antrmell 2/2 Phellinus ferrugineofuscus Phelferr 28/15 Antrodia pallescens Antrpall 39/22 Phellinus igniarius Pheligni 144/45 Antrodia serialis Antrseri 96/32 Phellinus laevigatus Phellaev 18/15 Antrodia sinuosa Antrsinu 15/11 Phellinus lundellii Phellund 13/6 Antrodia xantha Antrxant 8/7 Phellinus nigrolimitatus Phelnigr 16/6 Antrodiella faginea Antrfagi 1/1 Phellinus pini Phelpini 1/1 Antrodiella serpula Antrserp 12/7 Phellinus populicola Phelpopu 1/1 Aporpium canescens (NT) Protcary 2/2 Phellinus punctatus Phelpunc 58/16 Bjerkandera adusta Bjeradus 17/12 Phellinus tremulae Pheltrem 44/17 Butyrea luteoalba Steclute 6/6 Phellinus viticola Phelviti 36/20 Byssoporia mollicula Byssmoll 2/2 Physisporinus sanguinolentus Physsang 1/1 Canopora subfuscoflavida Cinelind 3/3 Physisporinus vitreus Physvitr 31/8 Ceriporia excelsa (NT) Ceriexce 3/2 Piptoporus betulinus Piptbetu 41/28 Ceriporia -

Investigating Wood Decaying Fungi Diversity in Central Siberia, Russia Using ITS Sequence Analysis and Interaction with Host Trees
sustainability Article Investigating Wood Decaying Fungi Diversity in Central Siberia, Russia Using ITS Sequence Analysis and Interaction with Host Trees Ji-Hyun Park 1, Igor N. Pavlov 2,3 , Min-Ji Kim 4, Myung Soo Park 1, Seung-Yoon Oh 5 , Ki Hyeong Park 1, Jonathan J. Fong 6 and Young Woon Lim 1,* 1 School of Biological Sciences and Institute of Microbiology, Seoul National University, Seoul 08826, Korea; [email protected] (J.-H.P.); [email protected] (M.S.P.); [email protected] (K.H.P.) 2 Laboratory of Reforestation, Mycology and Plant Pathology, V. N. Sukachev Institute of Forest, Siberian Branch of Russian Academy of Sciences, 660036 Krasnoyarsk, Russia; [email protected] 3 Department of Chemical Technology of Wood and Biotechnology, Reshetnev Siberian State University of Science and Technology, 660049 Krasnoyarsk, Russia 4 Wood Utilization Division, Forest Products Department, National Institute of Forest Science, Seoul 02455, Korea; [email protected] 5 Department of Biology and Chemistry, Changwon National University, Changwon 51140, Korea; [email protected] 6 Science Unit, Lingnan University, Tuen Mun, Hong Kong; [email protected] * Correspondence: [email protected]; Tel.: +82-2880-6708 Received: 27 February 2020; Accepted: 18 March 2020; Published: 24 March 2020 Abstract: Wood-decay fungi (WDF) play a significant role in recycling nutrients, using enzymatic and mechanical processes to degrade wood. Designated as a biodiversity hot spot, Central Siberia is a geographically important region for understanding the spatial distribution and the evolutionary processes shaping biodiversity. There have been several studies of WDF diversity in Central Siberia, but identification of species was based on morphological characteristics, lacking detailed descriptions and molecular data. -

Taxonomy and Phylogeny of Postia. Multi-Gene Phylogeny and Taxonomy of the Brown-Rot Fungi: Postia (Polyporales, Basidiomycota) and Related Genera
Persoonia 42, 2019: 101–126 ISSN (Online) 1878-9080 www.ingentaconnect.com/content/nhn/pimj RESEARCH ARTICLE https://doi.org/10.3767/persoonia.2019.42.05 Taxonomy and phylogeny of Postia. Multi-gene phylogeny and taxonomy of the brown-rot fungi: Postia (Polyporales, Basidiomycota) and related genera L.L. Shen1,2, M. Wang1, J.L. Zhou1, J.H. Xing1, B.K. Cui1,3,*, Y.C. Dai1,3,* Key words Abstract Phylogenetic and taxonomic studies on the brown-rot fungi Postia and related genera, are carried out. Phylogenies of these fungi are reconstructed with multiple loci DNA sequences including the internal transcribed Fomitopsidaceae spacer regions (ITS), the large subunit (nLSU) and the small subunit (nSSU) of nuclear ribosomal RNA gene, the multi-marker analyses small subunit of mitochondrial rRNA gene (mtSSU), the translation elongation factor 1-α gene (TEF1), the largest Oligoporus subunit of RNA polymerase II (RPB1) and the second subunit of RNA polymerase II (RPB2). Ten distinct clades of phylogeny Postia s.lat. are recognized. Four new genera, Amaropostia, Calcipostia, Cystidiopostia and Fuscopostia, are es- taxonomy tablished, and nine new species, Amaropostia hainanensis, Cyanosporus fusiformis, C. microporus, C. mongolicus, Tyromyces C. piceicola, C. subhirsutus, C. tricolor, C. ungulatus and Postia sublowei, are identified. Illustrated descriptions of wood-inhabiting fungi the new genera and species are presented. Identification keys to Postia and related genera, as well as keys to the species of each genus, are provided. Article info Received: 20 April 2017; Accepted: 28 September 2018; Published: 29 November 2018. INTRODUCTION Ryvarden & Gilbertson 1994, Núñez & Ryvarden 2001, Bernic- chia 2005, Ryvarden & Melo 2014). -

Three New Species of Postia (Aphyllophorales, Basidiomycota) from China
Fungal Diversity Three new species of Postia (Aphyllophorales, Basidiomycota) from China Yu-LianWei 1,2* and Yu-Cheng Dai1 1Institute of Applied Ecology, Chinese Academy of Sciences, Shenyang 110016, PR China 2Graduate School, Chinese Academy of Science, Beijing 100039, PR China Wei, Y.L. and Dai Y.C. (2006). Three new species of Postia (Aphyllophorales, Basidiomycota) from China. Fungal Diversity 23: 391-402. Three species of Postia (Aphyllophorales, Basidiomycota) from China are described as new. Postia calcarea is characterized by the pendent growth habit, chalky fruitbody, absence of cystidia, and by narrow and allantoid basidiospores. Postia gloeocystidiata has pileate basidiocarps with hispid upper surface, presence of gloeocystidia and hyphal pegs, and by narrowly cylindrical to allantoid basidiospores. Postia subundosa is distinguished from other species in the genus by its stipitate and pendent growth habit, cream to rust brown upper surface, large pores and hard corky to rigid context, brittle tubes, and by cylindrical to allantoid basidiospores. A key to Chinese species of Postia is provided. Key words: Polyporales, Postia calcarea, Postia gloeocystidiata, Postia subundosa, taxonomy, wood-rotting fungi. Introduction Postia Fr. (Aphyllophorales, Basidiomycota) is one of the important genera of brown rot fungi, and it is characterized by an annual growth habit, a monomitic hyphal system with clamp connections, thin-walled basidiospores, and the shapes of basidiospores mainly are ellipsoid, cylindrical to allantoid. Most of them are hyaline and negative in Melzer’s reagent and Cotton Blue. However, basidiospores in Postia caesia (Schrad.:Fr.) P. Karst. group are greyish to bluish (they are greyish in KOH), and they are weakly amyloid in Melzer’s reagent. -

Of the Białowieża Forest (Ne Poland)
Polish Botanical Journal 60(2): 217–292, 2015 DOI: 10.1515/pbj-2015-0034 AN ANNOTATED AND ILLUSTRATED CATALOGUE OF POLYPORES (AGARICOMYCETES) OF THE BIAŁOWIEŻA FOREST (NE POLAND) Dariusz Karasiński1 & Marek Wołkowycki Abstract. The Białowieża Forest (BF) is one of the best-preserved lowland deciduous and mixed forest complexes in Europe, rich in diverse fungi. This paper summarizes what is known about the poroid fungi of the Polish part of the Białowieża Forest, based on literature data, a re-examination of herbarium materials, and the authors’ studies from 1990–2014. An annotated catalogue of polypores recorded in the forest is presented, including 80 genera with 210 species. All literature and herbarium records are enumerated, and 160 species are illustrated with color pictures. Fourteen species previously reported in the literature have uncertain status because they lack voucher specimens and were not confirmed in recent field studies.Antrodiella subradula (Pilát) Niemelä & Miettinen, previously known from Asia, is reported for the first time from Europe. Fourteen species are newly reported from the Białowieża Forest (mainly from Białowieża National Park), including 8 species with first records in Poland (Antrodia hyalina Spirin, Miettinen & Kotir., Antrodia infirma Renvall & Niemelä, Antrodiella subradula, Junghuhnia fimbriatella (Peck) Ryvarden, Postia folliculocystidiata (Kotl. & Vampola) Niemelä & Vampola, Postia minusculoides (Pilát ex Pilát) Boulet, Skeletocutis chrysella Niemelä, Skeletocutis papyracea A. David), and 6 species reported previously from other localities in Poland [Antrodiella faginea Vampola & Pouzar, Dichomitus campestris (Quél.) Domański & Orlicz, Loweomyces fractipes (Berk. & M. A. Curtis) Jülich, Oxyporus latemarginatus (Durieu & Mont.) Donk, Perenniporia narymica (Pilát) Pouzar, Phellinus nigricans (Fr.) P. Karst.]. Several very rare European polypores already reported from the Białowieża Forest in the 20th century, such as Antrodia albobrunnea (Romell) Ryvarden, Antrodiella foliaceodentata (Nikol.) Gilb. -

Tremella Cetrariicola Diederich Et Coppins, Première Récolte En France
Bull. Ass. fr. lichénologie - 2015 - Vol. 40 - Fasc. 2 Tremella cetrariicola Diederich et Coppins, première récolte en France Pierre-Arthur Moreau1, Chantal Van Haluwyn2, Claude Roux3, Jean Michel Sussey4 1Département des sciences végétales et fongiques, Faculté des sciences pharmaceutiques et biologiques, EA 4483, Université de Lille, 3 rue du Pr Laguesse, 59006 Lille cedex 225 rue du Pévèle, 59113 Seclin 3Chemin des Vignes-Vieilles, 84120 Mirabeau 487 rue de la Pottaz, 74800 La Roche sur Foron Les trémelles (genre Tremella) sont des Basidiomycota (classe des Tremellomycetes) strictement parasites d’autres champignons lichénisés ou non, un caractère trophique ancestral chez les Basidiomycota et commun à la plupart des Tremellomycetes. Selon Diederich et al. (2014), 56 espèces de Tremella strictement lichénicoles ont été décrites, toutes spécifiques de leur hôte (espèce, genre, ou genres phylogénétiquement proches). Parmi les nombreuses espèces de lichens foliacés hébergeant des trémelles, figure Tuckermannopsis chlorophylla, hôte de Tremella cetrariicola Diederich et Coppins (in Diederich, 1996). L’un de nous (J.-M. S.) a récemment découvert cette trémelle en Haute-Savoie, alors qu’elle n’avait pas encore été mentionnée en France (Roux et al., 2014 : 1193). Description Récolte étudiée : France, Haute-Savoie, Mieussy, Sommand, forêt d’Ima, alt. 1450 m, sur Tuckermannopsis chlorophylla sur Picea abies, 2011/08/22, leg. et herb. J.-M. SUSSEY, det. C. ROUX, herb. P.-A. Moreau n° 11082200 (LIP). Les basidiomes sont superficiels, assez nombreux et disséminés sur les lobes du thalle. Ils se présentent sous la forme de protubérances de 0,5-2 mm de diamètre, plus ou moins resserrées à la base, variant du brun rouge au brun sombre, d’aspect cireux-pulpeux. -

The Fungal Tree of Life Genome-Scale Comparative
Guy Leonard & Thomas A. Richards Genome-scale comparative analysis of: Guy Leonard & Thomas A. Richards @guyleonard Synapomorphies To investigate the fidelity of gene fusion characters, we developed an approach for identifying A shared, derived character is used as a cladistic device, differentially distributed gene fusions among whole-genome datasets: fdfBLAST. grouping individual taxa or groups of taxa together into specific clades. Applying this tool to the Fungi, we identified 63 gene fusions present in two or more genomes. Using a combination of phylogenetic and comparative genomic analyses, A B A we then investigated the evolution of these genes across 115 The Fungal Tree of Life C fungal genomes, testing 36 C 12 Phanerochaete chrysosporium each gene fusion for 56 Phanerochaete carnosa A Accounting for these considerable sources of homoplasy, we identified 12 Phlebiopsis gigantea evidence of 16 31 12 36 Bjerkandera adusta B fusion characters that provide support for multiple nodes in the phylogeny 12 36 Phlebia brevispora 16 Fomitopsis pinicola A homoplasy, 32B 14 Postia placenta of the Fungi, including relationships within the deeply derived flagellum-forming 49 Agaricomycotina 12 Ceriporiopsis subvermispora B including gene 16 Wolfiporia cocos Agaricomycetes fungi (i.e. the chytrids) 32A 13 Ganoderma sp. B C Dichomitus squalens C 16 Basidiomycota fission, convergence, 32A 49 Trametes versicolor 13 16 49 8 12 16 12 Stereum hirsutum It has been argued that gene fission events occur at a low frequency because 36 Heterobasidion annosum Gene fusions can be used as synapomorphies if they are and horizontal 32B 36 56 38 45 Pleurotus ostreatus 12 15 16 32B 36 56 Laccaria bicolor shown to be stable and monophyletic; thus the root of a tree the process requires multiple simultaneous evolutionary occurances at 31 56 gene transfer.