Buckinghamshire Fungus Group Newsletter No. 13 August 2012
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Molecular Phylogenetic Studies in the Genus Amanita
1170 Molecular phylogenetic studies in the genus Amanita I5ichael Weiß, Zhu-Liang Yang, and Franz Oberwinkler Abstracl A group of 49 Amanita species that had been thoroughly examined morphologically and amtomically was analyzed by DNA sequence compadson to estimate natural groups and phylogenetic rclationships within the genus. Nuclear DNA sequences coding for a part of the ribosomal large subunit were determined and evaluated using neighbor-joining with bootstrap analysis, parsimony analysis, conditional clustering, and maximum likelihood methods, Sections Amanita, Caesarea, Vaginatae, Validae, Phalloideae, and Amidella were substantially confirmed as monophyletic groups, while the monophyly of section Lepidell.t remained unclear. Branching topologies between and within sections could also pafiially be derived. Stbgenera Amanita an'd Lepidella were not supported. The Mappae group was included in section Validae. Grouping hypotheses obtained by DNA analyses are discussed in relation to the distribution of morphological and anatomical chamcters in the studied species. Key words: fungi, basidiomycetes phylogeny, Agarrcales, Amanita systematics, large subunit rDNA, 28S. R6sum6 : A partir d'un groupe de 49 esp,ces d'Amanita prdalablement examinees morphologiquement et anatomiquement, les auteurs ont utilisd la comparaison des s€quences d'ADN pour ddfinir les groupes naturels et les relations phylog6ndtiques de ce genre. Les sdquences de I'ADN nucl6aire codant pour une partie de la grande sous-unit6 ribosomale ont 6t6 ddterminEes et €valu6es en utilisant l'analyse par liaison en lacet avec le voisin (neighbor-joining with bootstrap), l'analyse en parcimonie, le rcgroupement conditionnel et les m€thodes de ressemblance maximale. Les rdsultats confirment substantiellement les sections Afiarira, Caesarea, Uaqinatae, Ualidae, Phalloideae et Amidella, comme groupes monophyldtiques, alors que la monophylie de la section Lepidella demerxe obscure. -

Agaricales, Basidiomycota) Occurring in Punjab, India
Current Research in Environmental & Applied Mycology 5 (3): 213–247(2015) ISSN 2229-2225 www.creamjournal.org Article CREAM Copyright © 2015 Online Edition Doi 10.5943/cream/5/3/6 Ecology, Distribution Perspective, Economic Utility and Conservation of Coprophilous Agarics (Agaricales, Basidiomycota) Occurring in Punjab, India Amandeep K1*, Atri NS2 and Munruchi K2 1Desh Bhagat College of Education, Bardwal–Dhuri–148024, Punjab, India. 2Department of Botany, Punjabi University, Patiala–147002, Punjab, India. Amandeep K, Atri NS, Munruchi K 2015 – Ecology, Distribution Perspective, Economic Utility and Conservation of Coprophilous Agarics (Agaricales, Basidiomycota) Occurring in Punjab, India. Current Research in Environmental & Applied Mycology 5(3), 213–247, Doi 10.5943/cream/5/3/6 Abstract This paper includes the results of eco-taxonomic studies of coprophilous mushrooms in Punjab, India. The information is based on the survey to dung localities of the state during the various years from 2007-2011. A total number of 172 collections have been observed, growing as saprobes on dung of various domesticated and wild herbivorous animals in pastures, open areas, zoological parks, and on dung heaps along roadsides or along village ponds, etc. High coprophilous mushrooms’ diversity has been established and a number of rare and sensitive species recorded with the present study. The observed collections belong to 95 species spread over 20 genera and 07 families of the order Agaricales. The present paper discusses the distribution of these mushrooms in Punjab among different seasons, regions, habitats, and growing habits along with their economic utility, habitat management and conservation. This is the first attempt in which various dung localities of the state has been explored systematically to ascertain the diversity, seasonal availability, distribution and ecology of coprophilous mushrooms. -

Field Guide to Common Macrofungi in Eastern Forests and Their Ecosystem Functions
United States Department of Field Guide to Agriculture Common Macrofungi Forest Service in Eastern Forests Northern Research Station and Their Ecosystem General Technical Report NRS-79 Functions Michael E. Ostry Neil A. Anderson Joseph G. O’Brien Cover Photos Front: Morel, Morchella esculenta. Photo by Neil A. Anderson, University of Minnesota. Back: Bear’s Head Tooth, Hericium coralloides. Photo by Michael E. Ostry, U.S. Forest Service. The Authors MICHAEL E. OSTRY, research plant pathologist, U.S. Forest Service, Northern Research Station, St. Paul, MN NEIL A. ANDERSON, professor emeritus, University of Minnesota, Department of Plant Pathology, St. Paul, MN JOSEPH G. O’BRIEN, plant pathologist, U.S. Forest Service, Forest Health Protection, St. Paul, MN Manuscript received for publication 23 April 2010 Published by: For additional copies: U.S. FOREST SERVICE U.S. Forest Service 11 CAMPUS BLVD SUITE 200 Publications Distribution NEWTOWN SQUARE PA 19073 359 Main Road Delaware, OH 43015-8640 April 2011 Fax: (740)368-0152 Visit our homepage at: http://www.nrs.fs.fed.us/ CONTENTS Introduction: About this Guide 1 Mushroom Basics 2 Aspen-Birch Ecosystem Mycorrhizal On the ground associated with tree roots Fly Agaric Amanita muscaria 8 Destroying Angel Amanita virosa, A. verna, A. bisporigera 9 The Omnipresent Laccaria Laccaria bicolor 10 Aspen Bolete Leccinum aurantiacum, L. insigne 11 Birch Bolete Leccinum scabrum 12 Saprophytic Litter and Wood Decay On wood Oyster Mushroom Pleurotus populinus (P. ostreatus) 13 Artist’s Conk Ganoderma applanatum -

Biodiverse Master
Montane, Heath and Bog Habitats MONTANE, HEATH AND BOG HABITATS CONTENTS Montane, heath and bog introduction . 66 Opportunities for action in the Cairngorms . 66 The main montane, heath and bog biodiversity issues . 68 Main threats to UK montane, heath and bog Priority species in the Cairngorms . 72 UK Priority species and Locally important species accounts . 73 Cairngorms montane, heath and bog habitat accounts: • Montane . 84 • Upland heath . 87 • Blanket bog . 97 • Raised bog . 99 ‘Key’ Cairngorms montane, heath and bog species . 100 65 The Cairngorms Local Biodiversity Action Plan MONTANE, HEATH AND BOG INTRODUCTION Around one third of the Cairngorms Partnership area is over 600-650m above sea level (above the natural woodland line, although this is variable from place to place.). This comprises the largest and highest area of montane habitat in Britain, much of which is in a relatively pristine condition. It contains the main summits and plateaux with their associated corries, rocky cliffs, crags, boulder fields, scree slopes and the higher parts of some glens and passes. The vegeta- tion is influenced by factors such as exposure, snow cover and soil type. The main zone is considered to be one of the most spectacular mountain areas in Britain and is recognised nationally and internationally for the quality of its geology, geomorphology and topographic features, and associated soils and biodiversity. c14.5% of the Cairngorms Partnership area (75,000ha) is land above 600m asl. Upland heathland is the most extensive habitat type in the Cairngorms Partnership area, covering c41% of the area, frequently in mosaics with blanket bog. -

A Nomenclatural Study of Armillaria and Armillariella Species
A Nomenclatural Study of Armillaria and Armillariella species (Basidiomycotina, Tricholomataceae) by Thomas J. Volk & Harold H. Burdsall, Jr. Synopsis Fungorum 8 Fungiflora - Oslo - Norway A Nomenclatural Study of Armillaria and Armillariella species (Basidiomycotina, Tricholomataceae) by Thomas J. Volk & Harold H. Burdsall, Jr. Printed in Eko-trykk A/S, Førde, Norway Printing date: 1. August 1995 ISBN 82-90724-14-4 ISSN 0802-4966 A Nomenclatural Study of Armillaria and Armillariella species (Basidiomycotina, Tricholomataceae) by Thomas J. Volk & Harold H. Burdsall, Jr. Synopsis Fungorum 8 Fungiflora - Oslo - Norway 6 Authors address: Center for Forest Mycology Research Forest Products Laboratory United States Department of Agriculture Forest Service One Gifford Pinchot Dr. Madison, WI 53705 USA ABSTRACT Once a taxonomic refugium for nearly any white-spored agaric with an annulus and attached gills, the concept of the genus Armillaria has been clarified with the neotypification of Armillaria mellea (Vahl:Fr.) Kummer and its acceptance as type species of Armillaria (Fr.:Fr.) Staude. Due to recognition of different type species over the years and an extremely variable generic concept, at least 274 species and varieties have been placed in Armillaria (or in Armillariella Karst., its obligate synonym). Only about forty species belong in the genus Armillaria sensu stricto, while the rest can be placed in forty-three other modem genera. This study is based on original descriptions in the literature, as well as studies of type specimens and generic and species concepts by other authors. This publication consists of an alphabetical listing of all epithets used in Armillaria or Armillariella, with their basionyms, currently accepted names, and other obligate and facultative synonyms. -

Agarics-Stature-Types.Pdf
Gilled Mushroom Genera of Chicago Region, by stature type and spore print color. Patrick Leacock – June 2016 Pale spores = white, buff, cream, pale green to Pinkish spores Brown spores = orange, Dark spores = dark olive, pale lilac, pale pink, yellow to pale = salmon, yellowish brown, rust purplish brown, orange pinkish brown brown, cinnamon, clay chocolate brown, Stature Type brown smoky, black Amanitoid Amanita [Agaricus] Vaginatoid Amanita Volvariella, [Agaricus, Coprinus+] Volvopluteus Lepiotoid Amanita, Lepiota+, Limacella Agaricus, Coprinus+ Pluteotoid [Amanita, Lepiota+] Limacella Pluteus, Bolbitius [Agaricus], Coprinus+ [Volvariella] Armillarioid [Amanita], Armillaria, Hygrophorus, Limacella, Agrocybe, Cortinarius, Coprinus+, Hypholoma, Neolentinus, Pleurotus, Tricholoma Cyclocybe, Gymnopilus Lacrymaria, Stropharia Hebeloma, Hemipholiota, Hemistropharia, Inocybe, Pholiota Tricholomatoid Clitocybe, Hygrophorus, Laccaria, Lactarius, Entoloma Cortinarius, Hebeloma, Lyophyllum, Megacollybia, Melanoleuca, Inocybe, Pholiota Russula, Tricholoma, Tricholomopsis Naucorioid Clitocybe, Hygrophorus, Hypsizygus, Laccaria, Entoloma Agrocybe, Cortinarius, Hypholoma Lactarius, Rhodocollybia, Rugosomyces, Hebeloma, Gymnopilus, Russula, Tricholoma Pholiota, Simocybe Clitocyboid Ampulloclitocybe, Armillaria, Cantharellus, Clitopilus Paxillus, [Pholiota], Clitocybe, Hygrophoropsis, Hygrophorus, Phylloporus, Tapinella Laccaria, Lactarius, Lactifluus, Lentinus, Leucopaxillus, Lyophyllum, Omphalotus, Panus, Russula Galerinoid Galerina, Pholiotina, Coprinus+, -

Fruiting Body Form, Not Nutritional Mode, Is the Major Driver of Diversification in Mushroom-Forming Fungi
Fruiting body form, not nutritional mode, is the major driver of diversification in mushroom-forming fungi Marisol Sánchez-Garcíaa,b, Martin Rybergc, Faheema Kalsoom Khanc, Torda Vargad, László G. Nagyd, and David S. Hibbetta,1 aBiology Department, Clark University, Worcester, MA 01610; bUppsala Biocentre, Department of Forest Mycology and Plant Pathology, Swedish University of Agricultural Sciences, SE-75005 Uppsala, Sweden; cDepartment of Organismal Biology, Evolutionary Biology Centre, Uppsala University, 752 36 Uppsala, Sweden; and dSynthetic and Systems Biology Unit, Institute of Biochemistry, Biological Research Center, 6726 Szeged, Hungary Edited by David M. Hillis, The University of Texas at Austin, Austin, TX, and approved October 16, 2020 (received for review December 22, 2019) With ∼36,000 described species, Agaricomycetes are among the and the evolution of enclosed spore-bearing structures. It has most successful groups of Fungi. Agaricomycetes display great di- been hypothesized that the loss of ballistospory is irreversible versity in fruiting body forms and nutritional modes. Most have because it involves a complex suite of anatomical features gen- pileate-stipitate fruiting bodies (with a cap and stalk), but the erating a “surface tension catapult” (8, 11). The effect of gas- group also contains crust-like resupinate fungi, polypores, coral teroid fruiting body forms on diversification rates has been fungi, and gasteroid forms (e.g., puffballs and stinkhorns). Some assessed in Sclerodermatineae, Boletales, Phallomycetidae, and Agaricomycetes enter into ectomycorrhizal symbioses with plants, Lycoperdaceae, where it was found that lineages with this type of while others are decayers (saprotrophs) or pathogens. We constructed morphology have diversified at higher rates than nongasteroid a megaphylogeny of 8,400 species and used it to test the following lineages (12). -

Catalogue No. 121 – Sale, Special Offers and Recent Acquisitions
C. Arden, Bookseller Darren Bloodworth The Nursery, Forest Road, Hay-on-Wye, HR3 5DT, U.K. Tel: +44 (0) 1497-820471 Email: [email protected] Web: www.ardenbooks.co.uk Catalogue No. 121 – Sale, Special Offers and Recent Acquisitions Sale items : Botany 1 - 112 Entomology 113 - 140 Fine, Illustrated & Antiquarian 141 - 151 Gardening 152 - 207 General 208 - 254 Natural History & Zoology 255 - 266 New Naturalist s 267 - 302 Ornithology 303 - 346 Special offers : Botany 347 - 404 and recent Entomology 405 - 440 acquisitions Fine, Illustrated & Antiquarian 441 - 458 Gardening 459 - 512 Natural History & Zoology 513 - 562 New Naturalists 563 - 611 Ornithology 612 - 688 The stock in the Sale part of this catalogue (items 1 to 346) is an attempt to clear the remains of stock from the year’s previous catalogues. Book prices have already been reduced in many cases and further reductions are available to those who wish to take a risk that their chosen books will be available 10 or even 20 days after receiving this catalogue. Books will be dispatched once orders are complete – this may take up to three weeks if you order books at 50% off. How the Sale works First 10 days of sale…….All books available at prices shown in the catalogue After 10 days……………..If books are still available, we reduce their prices by 25% After 20 days……………..If books are still available, we reduce their prices by 50% We have also included over three hundred Special offers and recent acquisitions at the end of the catalogue (items 347 to 688). These Special offers and recent acquisitions are available at the prices indicated and are not part of the Sale terms. -

New Data on the Occurence of an Element Both
Analele UniversităĠii din Oradea, Fascicula Biologie Tom. XVI / 2, 2009, pp. 53-59 CONTRIBUTIONS TO THE KNOWLEDGE DIVERSITY OF LIGNICOLOUS MACROMYCETES (BASIDIOMYCETES) FROM CĂ3ĂğÂNII MOUNTAINS Ioana CIORTAN* *,,Alexandru. Buia” Botanical Garden, Craiova, Romania Corresponding author: Ioana Ciortan, ,,Alexandru Buia” Botanical Garden, 26 Constantin Lecca Str., zip code: 200217,Craiova, Romania, tel.: 0040251413820, e-mail: [email protected] Abstract. This paper presents partial results of research conducted between 2005 and 2009 in different forests (beech forests, mixed forests of beech with spruce, pure spruce) in CăSăĠânii Mountains (Romania). 123 species of wood inhabiting Basidiomycetes are reported from the CăSăĠânii Mountains, both saprotrophs and parasites, as identified by various species of trees. Keywords: diversity, macromycetes, Basidiomycetes, ecology, substrate, saprotroph, parasite, lignicolous INTRODUCTION MATERIALS AND METHODS The data presented are part of an extensive study, The research was conducted using transects and which will complete the PhD thesis. The CăSăĠânii setting fixed locations in some vegetable formations, Mountains are a mountain group of the ùureanu- which were visited several times a year beginning with Parâng-Lotru Mountains, belonging to the mountain the months April-May until October-November. chain of the Southern Carpathians. They are situated in Fungi were identified on the basis of both the SE parth of the Parâng Mountain, between OlteĠ morphological and anatomical properties of fruiting River in the west, Olt River in the east, Lotru and bodies and according to specific chemical reactions LaroriĠa Rivers in the north. Our area is 900 Km2 large using the bibliography [1-8, 10-13]. Special (Fig. 1). The vegetation presents typical levers: major presentation was made in phylogenetic order, the associations characteristic of each lever are present in system of classification used was that adopted by Kirk this massif. -

Three New Species of Cortinarius Subgenus Telamonia (Cortinariaceae, Agaricales) from China
A peer-reviewed open-access journal MycoKeys 69: 91–109 (2020) Three new species in Cortinarius from China 91 doi: 10.3897/mycokeys.69.49437 RESEARCH ARTICLE MycoKeys http://mycokeys.pensoft.net Launched to accelerate biodiversity research Three new species of Cortinarius subgenus Telamonia (Cortinariaceae, Agaricales) from China Meng-Le Xie1,2, Tie-Zheng Wei3, Yong-Ping Fu2, Dan Li2, Liang-Liang Qi4, Peng-Jie Xing2, Guo-Hui Cheng5,2, Rui-Qing Ji2, Yu Li2,1 1 Life Science College, Northeast Normal University, Changchun 130024, China 2 Engineering Research Cen- ter of Edible and Medicinal Fungi, Ministry of Education, Jilin Agricultural University, Changchun 130118, China 3 State Key Laboratory of Mycology, Institute of Microbiology, Chinese Academy of Sciences, Beijing 100101, China 4 Microbiology Research Institute, Guangxi Academy of Agriculture Sciences, Nanning, 530007, China 5 College of Plant Protection, Shenyang Agricultural University, Shenyang 110866, China Corresponding authors: Rui-Qing Ji ([email protected]), Yu Li ([email protected]) Academic editor: O. Raspé | Received 16 December 2019 | Accepted 23 June 2020 | Published 14 July 2020 Citation: Xie M-L, Wei T-Z, Fu Y-P, Li D, Qi L-L, Xing P-J, Cheng G-H, Ji R-Q, Li Y (2020) Three new species of Cortinarius subgenus Telamonia (Cortinariaceae, Agaricales) from China. MycoKeys 69: 91–109. https://doi. org/10.3897/mycokeys.69.49437 Abstract Cortinarius is an important ectomycorrhizal genus that forms a symbiotic relationship with certain trees, shrubs and herbs. Recently, we began studying Cortinarius in China and here we describe three new spe- cies of Cortinarius subg. Telamonia based on morphological and ecological characteristics, together with phylogenetic analyses. -

Lichens and Associated Fungi from Glacier Bay National Park, Alaska
The Lichenologist (2020), 52,61–181 doi:10.1017/S0024282920000079 Standard Paper Lichens and associated fungi from Glacier Bay National Park, Alaska Toby Spribille1,2,3 , Alan M. Fryday4 , Sergio Pérez-Ortega5 , Måns Svensson6, Tor Tønsberg7, Stefan Ekman6 , Håkon Holien8,9, Philipp Resl10 , Kevin Schneider11, Edith Stabentheiner2, Holger Thüs12,13 , Jan Vondrák14,15 and Lewis Sharman16 1Department of Biological Sciences, CW405, University of Alberta, Edmonton, Alberta T6G 2R3, Canada; 2Department of Plant Sciences, Institute of Biology, University of Graz, NAWI Graz, Holteigasse 6, 8010 Graz, Austria; 3Division of Biological Sciences, University of Montana, 32 Campus Drive, Missoula, Montana 59812, USA; 4Herbarium, Department of Plant Biology, Michigan State University, East Lansing, Michigan 48824, USA; 5Real Jardín Botánico (CSIC), Departamento de Micología, Calle Claudio Moyano 1, E-28014 Madrid, Spain; 6Museum of Evolution, Uppsala University, Norbyvägen 16, SE-75236 Uppsala, Sweden; 7Department of Natural History, University Museum of Bergen Allégt. 41, P.O. Box 7800, N-5020 Bergen, Norway; 8Faculty of Bioscience and Aquaculture, Nord University, Box 2501, NO-7729 Steinkjer, Norway; 9NTNU University Museum, Norwegian University of Science and Technology, NO-7491 Trondheim, Norway; 10Faculty of Biology, Department I, Systematic Botany and Mycology, University of Munich (LMU), Menzinger Straße 67, 80638 München, Germany; 11Institute of Biodiversity, Animal Health and Comparative Medicine, College of Medical, Veterinary and Life Sciences, University of Glasgow, Glasgow G12 8QQ, UK; 12Botany Department, State Museum of Natural History Stuttgart, Rosenstein 1, 70191 Stuttgart, Germany; 13Natural History Museum, Cromwell Road, London SW7 5BD, UK; 14Institute of Botany of the Czech Academy of Sciences, Zámek 1, 252 43 Průhonice, Czech Republic; 15Department of Botany, Faculty of Science, University of South Bohemia, Branišovská 1760, CZ-370 05 České Budějovice, Czech Republic and 16Glacier Bay National Park & Preserve, P.O. -
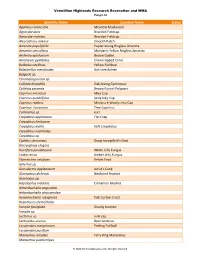
Scientific Name Common Name Status Agaricus Campestris
Vermillion Highlands Research Recreation and WMA Fungi List Scientific Name Common Name Status Agaricus campestris Meadow Mushroom Agrocybe dura Bearded Fieldcap Agrocybe molesta Bearded Fieldcap Aleurodiscus oakesii Smooth Patch Amanita populiphila Poplar-loving Ringless Amanita Amanita sinicoflava Mandarin Yellow Ringless Amanita Arrhenia epichysium Brown Goblet Artomyces pyxidatus Crown-tipped Coral Bolbitius vitellinus Yellow Fieldcap Boletinellus merulioides Ash-tree Bolete Bulgaria sp. Chromelosporium sp. Collybia dryophila Oak-loving Gymnopus Coltricia perennis Brown Funnel Polypore Coprinus micaceus Mica Cap Coprinus quadrifidus Scaly Inky Cap Coprinus radians Miniature Woolly Inky Cap Coprinus truncorum Tree Coprinus Cortinarius sp. cort Crepidotus applanatus Flat Crep Crepidotus herbarum Crepidotus mollis Soft Crepidotus Crepidotus nephrodes Crepidotus sp. Cyathus stercoreus Dung-loving Bird’s Nest Dacryopinax elegans Ductifera pululahuana White Jelly Fungus Exidia recisa Amber Jelly Fungus Flammulina velutipes Velvet Foot Galerina sp. Ganoderma applanatum Artist’s Conk Gloeoporus dichrous Bicolored Bracket Gymnopus sp. Hapalopilus nidulans Cinnamon Bracket Hohenbuehelia angustata Hohenbuehelia atrocaerulea Hymenochaete rubiginosa Oak Curtain Crust Hypomyces tremellicola Inocybe fastigiata Deadly Inocybe Inocybe sp. Lactarius sp. milk cap Lentinellus ursinus Bear Lentinus Lycoperdon marginatum Peeling Puffball Lycoperdon pusillum Marasmius oreades Fairy Ring Marasmius Marasmius pulcherripes © 2020 MinnesotaSeasons.com. All rights