Introduction to Cardiac Electrophysiology, the Electrocardiogram, and Cardiac Arrhythmias Alfred E
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Chapter 20 *Lecture Powerpoint the Circulatory System: Blood Vessels and Circulation
Chapter 20 *Lecture PowerPoint The Circulatory System: Blood Vessels and Circulation *See separate FlexArt PowerPoint slides for all figures and tables preinserted into PowerPoint without notes. Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Introduction • The route taken by the blood after it leaves the heart was a point of much confusion for many centuries – Chinese emperor Huang Ti (2697–2597 BC) believed that blood flowed in a complete circuit around the body and back to the heart – Roman physician Galen (129–c. 199) thought blood flowed back and forth like air; the liver created blood out of nutrients and organs consumed it – English physician William Harvey (1578–1657) did experimentation on circulation in snakes; birth of experimental physiology – After microscope was invented, blood and capillaries were discovered by van Leeuwenhoek and Malpighi 20-2 General Anatomy of the Blood Vessels • Expected Learning Outcomes – Describe the structure of a blood vessel. – Describe the different types of arteries, capillaries, and veins. – Trace the general route usually taken by the blood from the heart and back again. – Describe some variations on this route. 20-3 General Anatomy of the Blood Vessels Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Capillaries Artery: Tunica interna Tunica media Tunica externa Nerve Vein Figure 20.1a (a) 1 mm © The McGraw-Hill Companies, Inc./Dennis Strete, photographer • Arteries carry blood away from heart • Veins -

Glossary - Cellbiology
1 Glossary - Cellbiology Blotting: (Blot Analysis) Widely used biochemical technique for detecting the presence of specific macromolecules (proteins, mRNAs, or DNA sequences) in a mixture. A sample first is separated on an agarose or polyacrylamide gel usually under denaturing conditions; the separated components are transferred (blotting) to a nitrocellulose sheet, which is exposed to a radiolabeled molecule that specifically binds to the macromolecule of interest, and then subjected to autoradiography. Northern B.: mRNAs are detected with a complementary DNA; Southern B.: DNA restriction fragments are detected with complementary nucleotide sequences; Western B.: Proteins are detected by specific antibodies. Cell: The fundamental unit of living organisms. Cells are bounded by a lipid-containing plasma membrane, containing the central nucleus, and the cytoplasm. Cells are generally capable of independent reproduction. More complex cells like Eukaryotes have various compartments (organelles) where special tasks essential for the survival of the cell take place. Cytoplasm: Viscous contents of a cell that are contained within the plasma membrane but, in eukaryotic cells, outside the nucleus. The part of the cytoplasm not contained in any organelle is called the Cytosol. Cytoskeleton: (Gk. ) Three dimensional network of fibrous elements, allowing precisely regulated movements of cell parts, transport organelles, and help to maintain a cell’s shape. • Actin filament: (Microfilaments) Ubiquitous eukaryotic cytoskeletal proteins (one end is attached to the cell-cortex) of two “twisted“ actin monomers; are important in the structural support and movement of cells. Each actin filament (F-actin) consists of two strands of globular subunits (G-Actin) wrapped around each other to form a polarized unit (high ionic cytoplasm lead to the formation of AF, whereas low ion-concentration disassembles AF). -
Physiology Lessons for Use with the Biopac Student Lab Lesson 5
Physiology Lessons Lesson 5 for use with the ELECTROCARDIOGRAPHY I Biopac Student Lab Components of the ECG Richard Pflanzer, Ph.D. Associate Professor Emeritus Indiana University School of Medicine Purdue University School of Science William McMullen Vice President BIOPAC Systems, Inc. BIOPAC® Systems, Inc. 42 Aero Camino, Goleta, CA 93117 (805) 685-0066, Fax (805) 685-0067 Email: [email protected] Web: www.biopac.com Manual Revision PL3.7.5 03162009 BIOPAC Systems, Inc. Page 2 Biopac Student Lab 3.7.5 I. INTRODUCTION The main function of the heart is to pump blood through two circuits: 1. Pulmonary circuit: through the lungs to oxygenate the blood and remove carbon dioxide; and 2. Systemic circuit: to deliver oxygen and nutrients to tissues and remove carbon dioxide. Because the heart moves blood through two separate circuits, it is sometimes described as a dual pump. In order to beat, the heart needs three types of cells: 1. Rhythm generators, which produce an electrical signal (SA node or normal pacemaker); 2. Conductors to spread the pacemaker signal; and 3. Contractile cells (myocardium) to mechanically pump blood. The Electrical and Mechanical Sequence of a Heartbeat The heart has specialized pacemaker cells that start the electrical sequence of depolarization and repolarization. This property of cardiac tissue is called inherent rhythmicity or automaticity. The electrical signal is generated by the sinoatrial node (SA node) and spreads to the ventricular muscle via particular conducting pathways: internodal pathways and atrial fibers, the atrioventricular node (AV node), the bundle of His, the right and left bundle branches, and Purkinje fibers (Fig 5.1). -

Heart Rate Information Sheet Finding Your Pulse Taking Your Pulse
Heart rate In this activity you will measure your heart rate and investigate the effect that other things have on your heart rate. Information sheet You can measure your heart (pulse) rate anywhere on your body where a major artery is close to the surface of your skin. The easiest places are: • on the front of your forearm • just above your wrist on your thumb side • on the side of your neck about half way between your chin and your ear. Finding your pulse Try to locate your pulse in one of these places, using the tips of your index and middle fingers. You should feel a gentle, regular beat. This is your heart rate. Do not use your thumb, as your thumb has a pulse of its own. Taking your pulse When you find your pulse, use a stopwatch or a watch with a second hand to count how many beats there are in a full minute (60 seconds). In most situations, taking your pulse rate over one minute will give a reasonably accurate result, but if you want to take your pulse after exercise, you should do so over a much shorter time interval. After exercise your pulse rate will be changing rapidly. To get a reasonably accurate result, start to measure the rate immediately after the exercise and count the number of beats in 10 seconds. Multiply this number by six to find your heart rate. For an adult, a normal resting heart rate is between 60–100 beats a minute. The fitter you are, the lower your resting heart beat will be. -

Heart to Heart - STEAM Activity
Heart to Heart - STEAM Activity Purpose: The main objective of this exercise is to introduce how the human heart works. This lesson will be divided into four different lessons, including an introduction to heart anatomy, heart beats and pulses, and the circulatory system. We will be using coloring activities, stethoscopes, and handmade pumps to reinforce the concepts seen in this lesson. Vocabulary ● Artery: A blood vessel that carries blood high in oxygen content away from the heart to the farthest reaches of the body. ● Vein: a Blood vessel that carries blood low in oxygen content from the body back to the heart. ● Atrium: One of the two upper cavities of the heart that passes blood to the ventricles. ● Ventricles: One of the two lower chambers of the heart that receives blood from the atria. ● Valves: Tissue-paper thin membranes attached to the heart wall that constantly open and close to regulate blood flow. ● Pulse: A rhythmical, mechanical throbbing of the arteries as blood pumps through them. ● Heart Rate: The number of times per minute that the heart contracts - the number of heart beats per minute (bpm). ● Taquicardia: A high resting heart rate that is usually higher than 100 beats per minute. ● Bradycardia: A low resting heart rate that is usually lower than 60 beats per minute. ● Circulation: The movement of blood through the vessels of the body by the pumping action of the heart. It distributes nutrients and oxygen and removes waste products from all parts of the body. ● Pulmonary Circulation: The portion of the circulatory system that carries deoxygenated blood from the heart to the lungs and oxygenated blood back to the heart. -

Biopsychology 2012 – Sec 003 (Dr
Biopsychology 2012 – sec 003 (Dr. Campeau) Study Guide for First Midterm What are some fun facts about the human brain? - there are approximately 100 billion neurons in the brain; - each neuron makes between 1000 to 10000 connections with other neurons; - speed of action potentials varies from less than 1 mph and up to 100 mph. What is a neuron? A very specialized cell type whose function is to receive, process, and send information; these cells are found in the central nervous system (CNS – brain, spinal cord, retina) and the peripheral nervous system (PNS – the rest of the body). What is a nerve? They are axons of individual neurons in bundles or strands of many axons. What are the major parts of a neuron? - cell membrane: “skin” of the neuron; - cytoplasm: everything inside the skin; - nucleus: contains chromosomes (DNA); - ribosomes: generate proteins from mRNA; - mitochondria: energy “generator” of cells (produce ATP); - mitochondria: moves “stuff” inside the neuron (like a tow rope); - soma: cell body, excluding dendrites and axons; - dendrites and spines: part of the neuron usually receiving information from other neurons; - axons: part of the neuron that transmits information to other neurons; - myelin sheath: surrounds axons and provides electrical “insulation”; - nodes of Ranvier: small area on axons devoid of myelin sheath; - presynaptic terminal: area of neuron where neurotransmitter is stored and released by action potentials. What are the different neuron types according to function? 1. Sensory neurons: neurons specialized to “receive” information about the environment. 2. Motor neurons: neurons specialized to produce movement (contraction of muscles). 3. Interneurons or Intrinsic neurons: neurons, usually with short axons, that handle local information. -

Electrophysiology-Appnote.Pdf
Application Note: Electrophysiology Electrophysiology Introduction Electrophysiology is a field of research that deals with the electrical properties of cells and biological tissues. In some cases, it is used to test for nervous or cardiac diseases and abnormalities. In many research applications probes are used to measure the electrical activity of individual cells, tissues, and whole specimens. Measuring the activity of cells at various membrane potentials can give valuable information about ion transport mechanisms and cellular communication. Varying ionic strength and membrane potential on cell populations can be used to study contractile movements of muscle cells, and diseases affecting the normal propagation of impulses. Using various stimulation techniques along with a selection of quality equipment and setup design can give rise to a wealth of applications in electrophysiology. Electrophysiology Principle Researchers and clinicians use electrophysiology when studying the electrical properties of neural and muscle tissue. In the clinical laboratory, electroencephalograms are routinely performed as a test for neural disorders like epilepsy, brain tumor, stroke, encephalitis, and others by measuring the electrical activity of the brain through external electrodes. In the research laboratory, electrophysiology methods are used to measure the ion-channel activity of cell membranes in various electrical environments. Here, an extremely thin micropipette is used to make intimate contact with the cell membrane to study membrane potential. Neurons and other cell types derive their electrical properties from their lipid bilayer and the ion concentrations inside and outside of the cell. The ion concentration differences create a membrane potential difference on either side of the membrane. The flow of ions across the membrane generates a current that can be measured using Ohm’s law, where the change in voltage (V) is related to the current (I) and membrane resistance (R). -
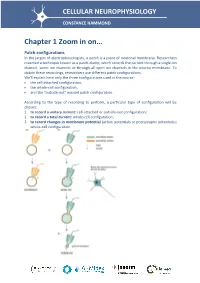
Chapter 1 Zoom in On… Patch Configurations in the Jargon of Electrophysiologists, a Patch Is a Piece of Neuronal Membrane
CELLULAR NEUROPHYSIOLOGY CONSTANCE HAMMOND Chapter 1 Zoom in on… Patch configurations In the jargon of electrophysiologists, a patch is a piece of neuronal membrane. Researchers invented a technique known as a patch-clamp, which records the current through a single ion channel, some ion channels or through all open ion channels in the neuron membrane. To obtain these recordings, researchers use different patch configurations. We'll explain here only the three configurations used in the course: the cell-attached configuration, the whole-cell configuration, and the "outside-out" excised patch configuration. According to the type of recording to perform, a particular type of configuration will be chosen: 1. to record a unitary current: cell-attached or outside-out configuration; 2. to record a total current: whole-cell configuration; 3. to record changes in membrane potential (action potentials or postsynaptic potentials): whole-cell configuration. The cell-attached configuration (attached to the pipette - Figure a) First, the pipette is filled with an extracellular fluid. Positive pressure is applied in the electrode by means of a syringe connected to the electrode, so that the intrapipette fluid tends to leave the pipette. The electrode is then brought near to the soma of a neuron. When the electrode touches the membrane, the positive pressure is withdrawn to draw the membrane toward the mouth of the pipette. We wait without moving the pipette; a seal is made between the walls of the pipette and the soma membrane. This seal strength can be measured by applying a low level of amplitude current in the pipette. Since V = RI, one can measure the resistance of the seal. -

Blood Vessels: Part A
Chapter 19 The Cardiovascular System: Blood Vessels: Part A Blood Vessels • Delivery system of dynamic structures that begins and ends at heart – Arteries: carry blood away from heart; oxygenated except for pulmonary circulation and umbilical vessels of fetus – Capillaries: contact tissue cells; directly serve cellular needs – Veins: carry blood toward heart Structure of Blood Vessel Walls • Lumen – Central blood-containing space • Three wall layers in arteries and veins – Tunica intima, tunica media, and tunica externa • Capillaries – Endothelium with sparse basal lamina Tunics • Tunica intima – Endothelium lines lumen of all vessels • Continuous with endocardium • Slick surface reduces friction – Subendothelial layer in vessels larger than 1 mm; connective tissue basement membrane Tunics • Tunica media – Smooth muscle and sheets of elastin – Sympathetic vasomotor nerve fibers control vasoconstriction and vasodilation of vessels • Influence blood flow and blood pressure Tunics • Tunica externa (tunica adventitia) – Collagen fibers protect and reinforce; anchor to surrounding structures – Contains nerve fibers, lymphatic vessels – Vasa vasorum of larger vessels nourishes external layer Blood Vessels • Vessels vary in length, diameter, wall thickness, tissue makeup • See figure 19.2 for interaction with lymphatic vessels Arterial System: Elastic Arteries • Large thick-walled arteries with elastin in all three tunics • Aorta and its major branches • Large lumen offers low resistance • Inactive in vasoconstriction • Act as pressure reservoirs—expand -

Central Venous Pressure: Uses and Limitations
Central Venous Pressure: Uses and Limitations T. Smith, R. M. Grounds, and A. Rhodes Introduction A key component of the management of the critically ill patient is the optimization of cardiovascular function, including the provision of an adequate circulating volume and the titration of cardiac preload to improve cardiac output. In spite of the appearance of several newer monitoring technologies, central venous pressure (CVP) monitoring remains in common use [1] as an index of circulatory filling and of cardiac preload. In this chapter we will discuss the uses and limitations of this monitor in the critically ill patient. Defining Central Venous Pressure What is the Central Venous Pressure? Central venous pressure is the intravascular pressure in the great thoracic veins, measured relative to atmospheric pressure. It is conventionally measured at the junction of the superior vena cava and the right atrium and provides an estimate of the right atrial pressure. The Central Venous Pressure Waveform The normal CVP exhibits a complex waveform as illustrated in Figure 1. The waveform is described in terms of its components, three ascending ‘waves’ and two descents. The a-wave corresponds to atrial contraction and the x descent to atrial relaxation. The c wave, which punctuates the x descent, is caused by the closure of the tricuspid valve at the start of ventricular systole and the bulging of its leaflets back into the atrium. The v wave is due to continued venous return in the presence of a closed tricuspid valve. The y descent occurs at the end of ventricular systole when the tricuspid valve opens and blood once again flows from the atrium into the ventricle. -

Brain Slice Preparation in Electrophysiology
Brain Slice Preparation In structural integrity, unlike cell cultures or tissue homogenates. Electrophysiology Some of the limitations of these preparations are: 1) lack of certain inputs and outputs normally existing in the Avital Schurr, Ph.D. intact brain; 2) certain portions of the sliced tissue, Department of Anesthesiology, especially the top and bottom surfaces of the slice, are University of Louisville School of Medicine, damaged by the slicing action itself; 3) the life span of a Louisville, Kentucky 40292 brain slice is limited and the tissue gets "older" at a much faster rate than the whole animal; 4) the effects of Avital Schurr is currently an Associate Professor in the decapitation ischemia on the viability of the slice are not Department of Anesthesiology at the University of well understood; 5) Since blood-borne factors may be Louisville. He received his Ph.D. in 1977 from Ben- missing from the artificial bathing medium of the brain Gurion University in Beer Sheva, Israel. From 1977 slice, they cannot benefit the preparation and thus the through 1981, he held two postdoctoral positions, one optimal composition of the bathing solution is not yet at the Baylor College of Medicine and the second at established. the University of Texas Medical School at Houston. Brain slice preparations are becoming PREPARATION OF SLICES increasingly popular among neurobiologists for the In general, rodents are the animals of choice for the study of the mammalian central nervous system preparation of brain slices. Of those, the rat and the guinea pig are the most used. After decapitation, the (CNS) in general and synaptic phenomena in brain is removed rapidly from the skull and rinsed with particular. -

Cardiovascular Physiology
CARDIOVASCULAR PHYSIOLOGY Ida Sletteng Karlsen • Trym Reiberg Second Edition 15 March 2020 Copyright StudyAid 2020 Authors Trym Reiberg Ida Sletteng Karlsen Illustrators Nora Charlotte Sønstebø Ida Marie Lisle Amalie Misund Ida Sletteng Karlsen Trym Reiberg Booklet Disclaimer All rights reserved. No part oF this book may be reproduced in any Form on by an electronic or mechanical means, without permission From StudyAid. Although the authors have made every efFort to ensure the inFormation in the booklet was correct at date of publishing, the authors do not assume and hereby disclaim any liability to any part For any inFormation that is omitted or possible errors. The material is taken From a variety of academic sources as well as physiology lecturers, but are Further incorporated and summarized in an original manner. It is important to note, the material has not been approved by professors of physiology. All illustrations in the booklet are original. This booklet is made especially For students at the Jagiellonian University in Krakow by tutors in the StudyAid group (students at JU). It is available as a PDF and is available for printing. If you have any questions concerning copyrights oF the booklet please contact [email protected]. About StudyAid StudyAid is a student organization at the Jagiellonian University in Krakow. Throughout the academic year we host seminars in the major theoretical subjects: anatomy, physiology, biochemistry, immunology, pathophysiology, supplementing the lectures provided by the university. We are a group of 25 tutors, who are students at JU, each with their own Field oF specialty. To make our seminars as useful and relevant as possible, we teach in an interactive manner often using drawings and diagrams to help students remember the concepts.