Flavonoids from Pseudotsuga Menziesii
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Vom Duxer Köpfl Bei Kufstein/Unterinntal, Österreich 129
48/49 Reihe A Series A/ Zitteliana An International Journal of Palaeontology and Geobiology Series A /Reihe A Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Geologie 48/49 An International Journal of Palaeontology and Geobiology München 2009 Zitteliana Zitteliana An International Journal of Palaeontology and Geobiology Series A/Reihe A Mitteilungen der Bayerischen Staatssammlung für Paläontologie und Geologie 48/49 CONTENTS/INHALT In memoriam † PROF. DR. VOLKER FAHLBUSCH 3 DHIRENDRA K. PANDEY, FRANZ T. FÜRSICH & ROSEMARIE BARON-SZABO Jurassic corals from the Jaisalmer Basin, western Rajasthan, India 13 JOACHIM GRÜNDEL Zur Kenntnis der Gattung Metriomphalus COSSMANN, 1916 (Gastropoda, Vetigastropoda) 39 WOLFGANG WITT Zur Ostracodenfauna des Ottnangs (Unteres Miozän) der Oberen Meeresmolasse Bayerns 49 NERIMAN RÜCKERT-ÜLKÜMEN Erstnachweis eines fossilen Vertreters der Gattung Naslavcea in der Türkei: Naslavcea oengenae n. sp., Untermiozän von Hatay (östliche Paratethys) 69 JÉRÔME PRIETO & MICHAEL RUMMEL The genus Collimys DAXNER-HÖCK, 1972 (Rodentia, Cricetidae) in the Middle Miocene fissure fillings of the Frankian Alb (Germany) 75 JÉRÔME PRIETO & MICHAEL RUMMEL Small and medium-sized Cricetidae (Mammalia, Rodentia) from the Middle Miocene fissure filling Petersbuch 68 (southern Germany) 89 JÉRÔME PRIETO & MICHAEL RUMMEL Erinaceidae (Mammalia, Erinaceomorpha) from the Middle Miocene fissure filling Petersbuch 68 (southern Germany) 103 JOSEF BOGNER The free-floating Aroids (Araceae) – living and fossil 113 RAINER BUTZMANN, THILO C. FISCHER & ERNST RIEBER Makroflora aus dem inneralpinen Fächerdelta der Häring-Formation (Rupelium) vom Duxer Köpfl bei Kufstein/Unterinntal, Österreich 129 MICHAEL KRINGS, NORA DOTZLER & THOMAS N. TAYLOR Globicultrix nugax nov. gen. et nov. spec. (Chytridiomycota), an intrusive microfungus in fungal spores from the Rhynie chert 165 MICHAEL KRINGS, THOMAS N. -

Rhizopogon Togasawariana Sp. Nov., the First Report of Rhizopogon Associated with an Asian Species of Pseudotsuga
Rhizopogon togasawariana sp. nov., the first report of Rhizopogon associated with an Asian species of Pseudotsuga Mujic, A. B., Hosaka, K., & Spatafora, J. W. (2014). Rhizopogon togasawariana sp. nov., the first report of Rhizopogon associated with an Asian species of Pseudotsuga. Mycologia, 106(1), 105-112. doi:10.3852/13-055 10.3852/13-055 Allen Press Inc. Version of Record http://hdl.handle.net/1957/47245 http://cdss.library.oregonstate.edu/sa-termsofuse Mycologia, 106(1), 2014, pp. 105–112. DOI: 10.3852/13-055 # 2014 by The Mycological Society of America, Lawrence, KS 66044-8897 Rhizopogon togasawariana sp. nov., the first report of Rhizopogon associated with an Asian species of Pseudotsuga Alija B. Mujic1 the natural and anthropogenic range of the family Department of Botany and Plant Pathology, Oregon and plays an important ecological role in the State University, Corvallis, Oregon 97331-2902 establishment and maintenance of forests (Tweig et Kentaro Hosaka al. 2007, Simard 2009). The foundational species Department of Botany, National Museum of Nature concepts for genus Rhizopogon were established in the and Science, Tsukuba-shi, Ibaraki, 305-0005, Japan North American monograph of Smith and Zeller (1966), and a detailed monograph also has been Joseph W. Spatafora produced for European Rhizopogon species (Martı´n Department of Botany and Plant Pathology, Oregon 1996). However, few data on Asian species of State University, Corvallis, Oregon 97331-2902 Rhizopogon have been incorporated into phylogenetic and taxonomic studies and only a limited account of Asian Rhizopogon species has been published for EM Abstract: Rhizopogon subgenus Villosuli are the only associates of Pinus (Hosford and Trappe 1988). -

Cedrus, Keteleeria, Pseudolarix, and Pseudo- Key Words: Abies, Larix
IAWA Journal, Vol. 15 (4), 1994: 399-406 FUSIFORM PARENCHYMA CELLS IN THE YOUNG WOOD OF PINACEAE, AND THEIR DISTINCTION FROM MARGINAL PARENCHYMA by Shuichi Noshiro and Tomoyuki Fujii Wood Anatomy Laboratory, Forestry and Forest Products Research Institute, Tsukuba Norin, P. O. Box 16, Ibaraki 305, Japan Summary Fusiform parenchyma cells found in sev ray tracheids, and the proportion of ray tra eral genera of Pinaceae are described and cheid pit border types. Recently, Anagnost compared with marginal parenchyma. Fusi et al. (1994) restudied all these features using form parenchyma cells are mostly fusiform world-wide sam pIes of Picea and Larix, and in shape, with occasional smooth horizontal confirmed the results of Bartholin (1979) that walls. They form discontinuous tangential ray tracheid pit border types were the most bands in complete or incomplete circ1es in the reliable characteristic. However, because of innermost growth rings of Larix, Abies, and restricted growth in branches, it was difficult Tsuga. Fusiform parenchyma always con to use these stemwood characteristics in iden tains resinous material, and is more conspic tifying branchwoods. uous in branchwoods than in stern woods. Modern branchwood materials gathered Marginal parenchyma cells were observed in for comparative purposes, however, revealed Cedrus, Keteleeria, Pseudolarix, and Pseudo the conspicuous occurrence of resinous paren tsuga as weIl as in Larix, Abies, and Tsuga, chyma cells in the inner growth rings of Larix and very rarely in Picea. Marginal paren and their absence in Picea. Occurrence of chyma cells are scattered along growth ring resinous parenchyma ceHs was also observed boundaries. They are always in strands with in the Quaternary branchwoods, and was used nodular horizontal walls with conspicuous to distinguish Larix branchwoods from Picea simple pits. -

Hiroyoshi OHASHI
J. Jpn. Bot. 91(5): 302–304 (2016) a, b c d Hiroyoshi OHASHI *, Tetsuo OHI-TOMA , Toshio KATSUKI and Nobuyuki TANAKA : Lectotype of Tsuga japonica (Pinaceae) aHerbarium TUS, Botanical Garden, Tohoku University, Sendai, 980-0862 JAPAN; bBotanical Gardens, Graduate School of Science, University of Tokyo, Bunkyo-ku, Tokyo, 112-0001 JAPAN; cTama Forest Science garden, Forestry and Forest Products research Institute, National research and Development Agency, Japan, 1833-81, Todori-machi, Hachioji, Tokyo, 193-0843 JAPAN; dDepartment of Botany, National Museum of Nature and Science, 4-1-1, Amakubo, Tsukuba, 305-0005 JAPAN *Corresponding author: [email protected] Summary: Tsuga japonica Shiras. is lectotypified and Wakayama Prefectures) in central Honshu by Plate III in the protologue published in Bot. and southeastern Kochi Pref. in Shikoku. The Mag. (Tokyo) 9(no. 96) in 1895. basionym of the name is Tsuga japonica Shiras. Pseudotsuga japonica (Shiras.) Beissn. It was published as “Tsuga (Pseudo-tsuga) is one of the remarkable endemic species japonica Shirasawa” in Bot. Mag. (Tokyo) 9(no. characterizing the flora of Japan. It occurs only 96): 41 (in Japanese) and 84 (in German) with in the southcentral Kii peninsula (Mie, Nara, Plate III in 1895 in the protologue. Pseudotsuga Fig. 1. Lectotype of Tsuga japonica Shiras. —302— October 2016 The Journal of Japanese Botany Vol. 91 No. 5 303 (as Pseudo-tsuga) in parenthesis is regarded as Shiras.’; Ohwi, Fl. Jap.: 42 (1953), & Fl. Jap. an infrageneric taxon of the genus Tsuga (ICN ed. Engl.: 112 (1965) as ‘Pseudotsuga japonica Art. 20.1, Rec. 21A.1 and Art. -

Ectomycorrhizal Fungal Communities in Endangered Pinus Amamiana Forests
RESEARCH ARTICLE Ectomycorrhizal fungal communities in endangered Pinus amamiana forests Masao Murata1*, Seiichi Kanetani2, Kazuhide Nara1 1 Graduate School of Frontier Sciences, The University of Tokyo, Kashiwa, Chiba, Japan, 2 Kyushu Research Center, Forestry and Forest Products Research Institute, Chuo-ku, Kumamoto, Japan * [email protected] a1111111111 a1111111111 Abstract a1111111111 a1111111111 Interactions between trees and ectomycorrhizal (ECM) fungi are critical for the growth and a1111111111 survival of both partners. However, ECM symbiosis in endangered trees has hardly been explored, complicating conservation efforts. Here, we evaluated resident ECM roots and soil spore banks of ECM fungi from endangered Pinus amamiana forests on Yakushima and Tanegashima Islands, Kagoshima Prefecture, Japan. Soil samples were collected OPEN ACCESS from remaining four forests in the two islands. The resident ECM roots in soil samples were subjected to molecular identification. Soil spore banks of ECM fungi were analyzed via bio- Citation: Murata M, Kanetani S, Nara K (2017) Ectomycorrhizal fungal communities in assays using a range of host seedlings (P. amamiana, P. parviflora, P. densiflora and Casta- endangered Pinus amamiana forests. PLoS ONE nopsis sieboldii) for 6±8 months. In all remaining P. amamiana forests, we discovered a new 12(12): e0189957. https://doi.org/10.1371/journal. Rhizopogon species (Rhizopogon sp.1), the sequence of which has no match amoung pone.0189957 numerous Rhizopogon sequences deposited in the international sequence database. Host Editor: Erika Kothe, Friedrich Schiller University, identification of the resident ECM roots confirmed that Rhizopogon sp.1 was associated GERMANY only with P. amamiana. Rhizopogon sp.1 was far more dominant in soil spore banks than in Received: August 27, 2017 resident ECM roots, and its presence was confirmed in nearly all soil samples examined Accepted: December 5, 2017 across the major remaining populations. -
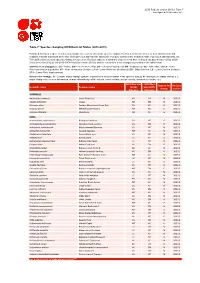
Table 7: Species Changing IUCN Red List Status (2012-2013)
IUCN Red List version 2013.2: Table 7 Last Updated: 25 November 2013 Table 7: Species changing IUCN Red List Status (2012-2013) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2012 (IUCN Red List version 2012.2) and 2013 (IUCN Red List version 2013.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2012) List (2013) change version Category Category MAMMALS Nycticebus javanicus Javan Slow Loris EN CR N 2013.2 Okapia johnstoni Okapi NT EN N 2013.2 Pteropus niger Greater Mascarene Flying -

Page 1 the Following Pages Outline Phasing Recommendations for The
Kruckeberg Botanic Garden Master Site Plan PHASING AND IMPLEMENTATION he following pages outline phasing recommendations for the KruckebergT Botanic Garden that seem desirable to address the needs, vision, and requirements of a private garden’s evolution into the publc domain. With the transfer of this property from a private residence to a commercial public entity, new sets of codes, restrictions, and opportunities come into play. These deal with public safety, health, and well-being and ensure that equal opportunities are afforded to all. Within a limited budget, Phase 1 responds to these immediate needs by providing on-site public parking to reduce impacts to the surrounding residential community, adding much needed public restrooms, and creating a permanent and separate service access road and staff parking area. Phase 2 focuses on siting an interpretive switchback boardwalk trail that connects the upper and lower gardens in an aesthetic ADA-compliant manner. It is also envi- sioned that an ADA-compliant loop path would be routed through the lower garden. While it would be optimal to build the environmental learning center in Phase 2, it is recognized that lack of funding may require deferment to a later phase. Further development of future phases depends on many factors, most importantly securing funding and the commitment of the City, Foundation, and public to sup- port and encourage new work to proceed. In the end, this alone will determine how quickly Garden projects are completed and the Garden vision, as outlined in this report, is realized. This is a modest plan as represented by the development costs associated with each phase in 2010 dollars. -

IAWA List of Microscopic Features for Softwood Identification 1
IAWA List of microscopic features for softwood identification 1 IAWA LIST OF MICROSCOPIC FEATURES FOR SOFTWOOD IDENTIFICATION IAWA Committee Pieter Baas – Leiden, The Netherlands Nadezhda Blokhina – Vladivostok, Russia Tomoyuki Fujii – Ibaraki, Japan Peter Gasson – Kew, UK Dietger Grosser – Munich, Germany Immo Heinz – Munich, Germany Jugo Ilic – South Clayton, Australia Jiang Xiaomei – Beijing, China Regis Miller – Madison, WI, USA Lee Ann Newsom – University Park, PA, USA Shuichi Noshiro – Ibaraki, Japan Hans Georg Richter – Hamburg, Germany Mitsuo Suzuki – Sendai, Japan Teresa Terrazas – Montecillo, Mexico Elisabeth Wheeler – Raleigh, NC, USA Alex Wiedenhoeft – Madison, WI, USA Edited by H.G. Richter, D. Grosser, I. Heinz & P.E. Gasson © 2004. IAWA Journal 25 (1): 1–70 Published for the International Association of Wood Anatomists at the Nationaal Herbarium Nederland, Leiden, The Netherlands Downloaded from Brill.com10/05/2021 05:46:26PM via free access 2 IAWA Journal, Vol. 25 (1), 2004 IAWA List of microscopic features for softwood identification 3 Downloaded from Brill.com10/05/2021 05:46:26PM via free access 2 IAWA Journal, Vol. 25 (1), 2004 IAWA List of microscopic features for softwood identification 3 PREFACE A definitive list of anatomical features of softwoods has long been needed. The hard- wood list (IAWA Committee 1989) has been adopted throughout the world, not least because it provides a succinct, unambiguous illustrated glossary of hardwood charac- ters that can be used for a variety of purposes, not just identification. This publication is intended to do the same job for softwoods. Identifying softwoods relies on careful observation of a number of subtle characters, and great care has been taken to show high quality photomicrographs that remove most of the ambiguity that definitions alone would provide. -

Polly Hill Arboretum Plant Collection Inventory March 14, 2011 *See
Polly Hill Arboretum Plant Collection Inventory March 14, 2011 Accession # Name COMMON_NAME Received As Location* Source 2006-21*C Abies concolor White Fir Plant LMB WEST Fragosa Landscape 93-017*A Abies concolor White Fir Seedling ARB-CTR Wavecrest Nursery 93-017*C Abies concolor White Fir Seedling WFW,N1/2 Wavecrest Nursery 2003-135*A Abies fargesii Farges Fir Plant N Morris Arboretum 92-023-02*B Abies firma Japanese Fir Seed CR5 American Conifer Soc. 82-097*A Abies holophylla Manchurian Fir Seedling NORTHFLDW Morris Arboretum 73-095*A Abies koreana Korean Fir Plant CR4 US Dept. of Agriculture 73-095*B Abies koreana Korean Fir Plant ARB-W US Dept. of Agriculture 97-020*A Abies koreana Korean Fir Rooted Cutting CR2 Jane Platt 2004-289*A Abies koreana 'Silberlocke' Korean Fir Plant CR1 Maggie Sibert 59-040-01*A Abies lasiocarpa 'Martha's Vineyard' Arizona Fir Seed ARB-E Longwood Gardens 59-040-01*B Abies lasiocarpa 'Martha's Vineyard' Arizona Fir Seed WFN,S.SIDE Longwood Gardens 64-024*E Abies lasiocarpa var. arizonica Subalpine Fir Seedling NORTHFLDE C. E. Heit 2006-275*A Abies mariesii Maries Fir Seedling LNNE6 Morris Arboretum 2004-226*A Abies nephrolepis Khingan Fir Plant CR4 Morris Arboretum 2009-34*B Abies nordmanniana Nordmann Fir Plant LNNE8 Morris Arboretum 62-019*A Abies nordmanniana Nordmann Fir Graft CR3 Hess Nursery 62-019*B Abies nordmanniana Nordmann Fir Graft ARB-CTR Hess Nursery 62-019*C Abies nordmanniana Nordmann Fir Graft CR3 Hess Nursery 62-028*A Abies nordmanniana Nordmann Fir Plant ARB-W Critchfield Tree Fm 95-029*A Abies nordmanniana Nordmann Fir Seedling NORTHFLDN Polly Hill Arboretum 86-046*A Abies nordmanniana ssp. -

UNIVERSITÀ DEGLI STUDI DI PADOVA FACOLTÀ DI AGRARIA Dipartimento Territorio E Sistemi Agro-Forestali LAUREA MAGISTRALE in SCIENZE FORESTALI E AMBIENTALI
UNIVERSITÀ DEGLI STUDI DI PADOVA FACOLTÀ DI AGRARIA Dipartimento Territorio e Sistemi Agro-forestali LAUREA MAGISTRALE IN SCIENZE FORESTALI E AMBIENTALI IL GENERE PSEUDOTSUGA E LA SELVICOLTURA DELL’ ABETE DI DOUGLAS (PSEUDOTSUGA MENZIESII VAR. MENZIESII (MIRB.) FRANCO) IN ITALIA: UN APPROCCIO ECOLOGICO THE GENUS PSEUDOTSUGA AND THE SILVICULTURE OF DOUGLAS- FIR (PSEUDOTSUGA MENZIESII VAR. MENZIESII (MIRB.) FRANCO) IN ITALY: AN ECOLOGICAL APPROACH Relatore Laureando Prof. Pividori Mario Villa Jona (1180240) Anno Accademico 2019 – 2020 “Alla mia famiglia, e in particolare a mia nonna Maria Lorena” Illustrazione di Pseudotsuga menziesii var. menziesii nella Catena della Cascate (Stati Uniti occidentali). Riproduzione di Jim Stevenson ridisegnata da Villa Jona Indice 0. Introduzione 3 1. Il genere Pseudotsuga 4 1.1 Etimologia del genere Pseudotsuga 5 2. Storia fossile, paleobotanica ed evoluzione 6 2.1 Fossili in Nord America 10 2.2 Fossili in Europa 17 2.3 Fossili in Asia 18 2.4 Evoluzione e speciazione 19 3. Tassonomia del genere Pseudotsuga 21 3.1 Le specie odierne 23 3.2 Conservazione 39 4. L’Abete di Douglas (Pseudotsuga menziesii (Mirb.) Franco) 41 4.1 Descrizione 41 4.1.2 Nome scientifico 48 4.1.3 Nome comune 50 4.2 Le varietà di Douglasia: storia e inquadramento botanico 51 4.3 Corologia e distribuzione 54 4.4 Ecologia 57 4.5 Esigenze pedoclimatiche 65 4.5.1 Suolo 68 5. Valenza xilologica 69 6. La selvicoltura della Douglasia 72 6.1 Il fallimento nel contesto italiano 83 7. Curiosità 85 8. Conclusioni 85 9. Bibliografia 86 9.1 Libri 96 9.2 Convegni 96 9.3 Sitografia 96 1 Riassunto (Italiano) Questa tesi si pone lo scopo di ripercorrere la storia, la biologia, l’ecologia e la selvicoltura (in Nord America ed Europa) di una delle specie forestali più importanti al mondo, Pseudotsuga menziesii (Mirb.) Franco, e del suo genere di appartenenza, con un particolare focus alla situazione italiana odierna e passata. -

Enantiomeric Natural Products: Occurrence and Biogenesis Jennifer M
Angewandte. Reviews R. M. Williams et al. DOI: 10.1002/anie.201107204 Natural Enantiomers Enantiomeric Natural Products: Occurrence and Biogenesis Jennifer M. Finefield, David H. Sherman, Martin Kreitman, and Robert M. Williams* Keywords: biosynthesis · enantioselectivity · isolation · natural products Angewandte Chemie 4802 www.angewandte.org 2012 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim Angew. Chem. Int. Ed. 2012, 51, 4802 – 4836 Angewandte Enantiomeric Natural Products Chemie In nature, chiral natural products are usually produced in optically From the Contents pure form—however, occasionally both enantiomers are formed. These enantiomeric natural products can arise from a single species or 1. Introduction 4803 from different genera and/or species. Extensive research has been 2. Terpenes 4803 carried out over the years in an attempt to understand the biogenesis of naturally occurring enantiomers; however, many fascinating puzzles 3. Phenylpropanoids 4810 and stereochemical anomalies still remain. 4. Polyketides 4816 5. Alkaloids 4821 1. Introduction 6. Summary and Outlook 4829 Terrestrial and marine plants, animals, fungi, and bacteria (among others) are known to produce a multitude of secondary metabolites, often referred to as “natural prod- ucts.”[1] In contrast to the required production of primary overwhelming number of known secondary metabolites, and metabolites to sustain life, organisms can generally survive the often overlooked reporting of the optical rotation or CD without the production of secondary metabolites; however, -
Japanese Plants in UK Gardens
Conservation of threatened Japanese plants in UK gardens Ildikó Whitton and Suzanne Sharrock Conservation of threatened Japanese plants in UK gardens By Ildikó Whitton and Suzanne Sharrock August 2011 Recommended citation: Whitton, I. and Sharrock, S., 2011. Conservation of threatened Japanese plants in UK gardens. Botanic Gardens Conservation International, Richmond, UK. ISBN: 978-1-905164-36-3 Published by Botanic Gardens Conservation International Descanso House, 199 Kew Road, Richmond, Surrey, TW9 3BW, UK Front cover image: Rosa hirtula (Ildikó Whitton) Back cover image: Bletilla striata (John Anderson) Design: John Morgan, Seacape. www.seascapedesign.co.uk Acknowledgements This project was supported by the Daiwa Anglo-Japanese Foundation. BGCI is grateful for the efforts made by botanic gardens around the world to supply plant data to the PlantSearch database. We also acknowledge the contribution of the participating institutions that responded to our survey and provided information about their collections, especially our case study contributors. Particular thanks are due to Owen Johnson (The Tree Register) and Noelia Alvarez (Royal Botanic Gardens, Kew) for providing additional data, Meirion Jones at BGCI for assistance with data analysis, and Junko Katayama for translation into Japanese and coordination of contacts with the Japanese Association of Botanic Gardens. Botanic Gardens Conservation International (BGCI) Linking more than 800 botanic gardens and other partners in some 120 countries, BGCI forms the world’s largest plant conservation network. From grass-roots action to global policy development, BGCI operates at all levels to achieve plant conservation, environmental education and development goals. We aim to ensure that plants are recognised as one of the world’s most important natural resources, providing essential ecosystem services and underpinning all life on Earth.