A Survey of Butterflies from Aruba and Bonaire and New Records for Curaçao
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Ix Viii the World by Income
The world by income Classified according to World Bank estimates of 2016 GNI per capita (current US dollars,Atlas method) Low income (less than $1,005) Greenland (Den.) Lower middle income ($1,006–$3,955) Upper middle income ($3,956–$12,235) Faroe Russian Federation Iceland Islands High income (more than $12,235) (Den.) Finland Norway Sweden No data Canada Netherlands Estonia Isle of Man (U.K.) Russian Latvia Denmark Fed. Lithuania Ireland U.K. Germany Poland Belarus Belgium Channel Islands (U.K.) Ukraine Kazakhstan Mongolia Luxembourg France Moldova Switzerland Romania Uzbekistan Dem.People’s Liechtenstein Bulgaria Georgia Kyrgyz Rep.of Korea United States Azer- Rep. Spain Monaco Armenia Japan Portugal Greece baijan Turkmenistan Tajikistan Rep.of Andorra Turkey Korea Gibraltar (U.K.) Syrian China Malta Cyprus Arab Afghanistan Tunisia Lebanon Rep. Iraq Islamic Rep. Bermuda Morocco Israel of Iran (U.K.) West Bank and Gaza Jordan Bhutan Kuwait Pakistan Nepal Algeria Libya Arab Rep. Bahrain The Bahamas Western Saudi Qatar Cayman Is. (U.K.) of Egypt Bangladesh Sahara Arabia United Arab India Hong Kong, SAR Cuba Turks and Caicos Is. (U.K.) Emirates Myanmar Mexico Lao Macao, SAR Haiti Cabo Mauritania Oman P.D.R. N. Mariana Islands (U.S.) Belize Jamaica Verde Mali Niger Thailand Vietnam Guatemala Honduras Senegal Chad Sudan Eritrea Rep. of Guam (U.S.) Yemen El Salvador The Burkina Cambodia Philippines Marshall Nicaragua Gambia Faso Djibouti Federated States Islands Guinea Benin Costa Rica Guyana Guinea- Brunei of Micronesia Bissau Ghana Nigeria Central Ethiopia Sri R.B. de Suriname Côte South Darussalam Panama Venezuela Sierra d’Ivoire African Lanka French Guiana (Fr.) Cameroon Republic Sudan Somalia Palau Colombia Leone Togo Malaysia Liberia Maldives Equatorial Guinea Uganda São Tomé and Príncipe Rep. -

The Value of Nature in the Caribbean Netherlands
The Economics of Ecosystems The value of nature and Biodiversity in the Caribbean Netherlands in the Caribbean Netherlands 2 Total Economic Value in the Caribbean Netherlands The value of nature in the Caribbean Netherlands The Challenge Healthy ecosystems such as the forests on the hillsides of the Quill on St Eustatius and Saba’s Mt Scenery or the corals reefs of Bonaire are critical to the society of the Caribbean Netherlands. In the last decades, various local and global developments have resulted in serious threats to these fragile ecosystems, thereby jeopardizing the foundations of the islands’ economies. To make well-founded decisions that protect the natural environment on these beautiful tropical islands against the looming threats, it is crucial to understand how nature contributes to the economy and wellbeing in the Caribbean Netherlands. This study aims to determine the economic value and the societal importance of the main ecosystem services provided by the natural capital of Bonaire, St Eustatius and Saba. The challenge of this project is to deliver insights that support decision-makers in the long-term management of the islands’ economies and natural environment. Overview Caribbean Netherlands The Caribbean Netherlands consist of three islands, Bonaire, St Eustatius and Saba all located in the Caribbean Sea. Since 2010 each island is part of the Netherlands as a public entity. Bonaire is the largest island with 16,000 permanent residents, while only 4,000 people live in St Eustatius and approximately 2,000 in Saba. The total population of the Caribbean Netherlands is 22,000. All three islands are surrounded by living coral reefs and therefore attract many divers and snorkelers. -
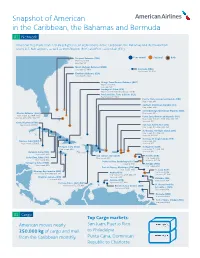
Snapshot of American in the Caribbean, the Bahamas and Bermuda 01 Network
Snapshot of American in the Caribbean, the Bahamas and Bermuda 01 Network American flies more than 170 daily flights to 38 destinations in the Caribbean, the Bahamas and Bermuda from seven U.S. hub airports, as well as from Boston (BOS) and Fort Lauderdale (FLL). Freeport, Bahamas (FPO) Year-round Seasonal Both Year-round: MIA Seasonal: CLT Marsh Harbour, Bahamas (MHH) Seasonal: CLT, MIA Bermuda (BDA) Year-round: JFK, PHL Eleuthera, Bahamas (ELH) Seasonal: CLT, MIA George Town/Exuma, Bahamas (GGT) Year-round: MIA Seasonal: CLT Santiago de Cuba (SCU) Year-round: MIA (Coming May 3, 2019) Providenciales, Turks & Caicos (PLS) Year-round: CLT, MIA Puerto Plata, Dominican Republic (POP) Year-round: MIA Santiago, Dominican Republic (STI) Year-round: MIA Santo Domingo, Dominican Republic (SDQ) Nassau, Bahamas (NAS) Year-round: MIA Year-round: CLT, MIA, ORD Punta Cana, Dominican Republic (PUJ) Seasonal: DCA, DFW, LGA, PHL Year-round: BOS, CLT, DFW, MIA, ORD, PHL Seasonal: JFK Grand Cayman (GCM) Year-round: CLT, MIA San Juan, Puerto Rico (SJU) Year-round: CLT, MIA, ORD, PHL St. Thomas, US Virgin Islands (STT) Year-round: CLT, MIA, SJU Seasonal: JFK, PHL St. Croix, US Virgin Islands (STX) Havana, Cuba (HAV) Year-round: MIA Year-round: CLT, MIA Seasonal: CLT Holguin, Cuba (HOG) St. Maarten (SXM) Year-round: MIA Year-round: CLT, MIA, PHL Varadero, Cuba (VRA) Seasonal: JFK Year-round: MIA Cap-Haïtien, Haiti (CAP) St. Kitts (SKB) Santa Clara, Cuba (SNU) Year-round: MIA Year-round: MIA Year-round: MIA Seasonal: CLT, JFK Pointe-a-Pitre, Guadeloupe (PTP) Camagey, Cuba (CMW) Seasonal: MIA Antigua (ANU) Year-round: MIA Year-round: MIA Fort-de-France, Martinique (FDF) Year-round: MIA St. -

St. Lucia Earns Junior Chef Award at Taste Of
MEDIA CONTACTS: KTCpr Theresa M. Oakes / [email protected] Leigh-Mary Hoffmann / [email protected] Telephone: 516-594-4100 #1101 **high resolution images available** BAHAMAS WINS TEAM, BARTENDER, PASTRY TOP HONORS; PUERTO RICO TAKES CHEF CATEGORY; ST. LUCIA EARNS JUNIOR CHEF AWARD AT TASTE OF THE CARIBBEAN 2015 MIAMI, FL (June 15, 2015) – The Bahamas National Culinary Team won three of the five top categories at the Taste of the Caribbean culinary competition this past weekend earning honors as Caribbean National Team of the Year and individual honors to Marv Cunningham, Bahamas for Caribbean Bartender of the Year and four-time winner Sheldon Tracey Sweeting, Bahamas, for Caribbean Pastry Chef of the Year. Jonathan Hernandez, Puerto Rico was crowned Caribbean Chef of the Year and Edna Butcher, St. Lucia, was named Caribbean Junior Chef of the Year. "Congratulations to all of the Taste of the Caribbean participants, their national hotel associations, team managers and sponsors for developing 10 Caribbean national culinary teams to compete at our annual culinary event," said Frank Comito, director general and CEO of the Caribbean Hotel and Tourism Association (CHTA). "Your commitment to culinary excellence in our region is very much appreciated as we showcase the region’s culinary and beverage offerings. Congratulations to all of the winners for a job well-done," Comito added. Presented by CHTA, Taste of the Caribbean featured cooking and bartending competitions between 10 Caribbean culinary teams from Anguilla, Bahamas, Barbados, Bonaire, British Virgin Islands, Jamaica, Puerto Rico, St. Lucia, Suriname and the U.S. Virgin Islands with the winners being named the "best of the best" throughout the region. -

Origins of Six Species of Butterflies Migrating Through Northeastern
diversity Article Origins of Six Species of Butterflies Migrating through Northeastern Mexico: New Insights from Stable Isotope (δ2H) Analyses and a Call for Documenting Butterfly Migrations Keith A. Hobson 1,2,*, Jackson W. Kusack 2 and Blanca X. Mora-Alvarez 2 1 Environment and Climate Change Canada, 11 Innovation Blvd., Saskatoon, SK S7N 0H3, Canada 2 Department of Biology, University of Western Ontario, Ontario, ON N6A 5B7, Canada; [email protected] (J.W.K.); [email protected] (B.X.M.-A.) * Correspondence: [email protected] Abstract: Determining migratory connectivity within and among diverse taxa is crucial to their conservation. Insect migrations involve millions of individuals and are often spectacular. However, in general, virtually nothing is known about their structure. With anthropogenically induced global change, we risk losing most of these migrations before they are even described. We used stable hydrogen isotope (δ2H) measurements of wings of seven species of butterflies (Libytheana carinenta, Danaus gilippus, Phoebis sennae, Asterocampa leilia, Euptoieta claudia, Euptoieta hegesia, and Zerene cesonia) salvaged as roadkill when migrating in fall through a narrow bottleneck in northeast Mexico. These data were used to depict the probabilistic origins in North America of six species, excluding the largely local E. hegesia. We determined evidence for long-distance migration in four species (L. carinenta, E. claudia, D. glippus, Z. cesonia) and present evidence for panmixia (Z. cesonia), chain (Libytheana Citation: Hobson, K.A.; Kusack, J.W.; Mora-Alvarez, B.X. Origins of Six carinenta), and leapfrog (Danaus gilippus) migrations in three species. Our investigation underlines Species of Butterflies Migrating the utility of the stable isotope approach to quickly establish migratory origins and connectivity in through Northeastern Mexico: New butterflies and other insect taxa, especially if they can be sampled at migratory bottlenecks. -

Bonaire National Marine Park Netherlands
UNITED NATIONS EP United Nations Original: ENGLISH Environment Program Proposed areas for inclusion in the SPAW list ANNOTATED FORMAT FOR PRESENTATION REPORT FOR: Bonaire National Marine Park Netherlands Date when making the proposal : October 5th, 2010 CRITERIA SATISFIED : Ecological criteria Cultural and socio-economic criteria Representativeness Cultural and traditional use Diversity Area name: Bonaire National Marine Park Country: Netherlands Contacts Last name: HOETJES First name: Paul Focal Point Position: Policy Coordinator Nature Email: [email protected] Phone: (+599) 715 83 08 Last name: De Leon First name: Ramón Manager Position: Park Manager Email: [email protected] Phone: + 599 717 8444 SUMMARY Chapter 1 - IDENTIFICATION Chapter 2 - EXECUTIVE SUMMARY Chapter 3 - SITE DESCRIPTION Chapter 4 - ECOLOGICAL CRITERIA Chapter 5 - CULTURAL AND SOCIO-ECONOMIC CRITERIA Chapter 6 - MANAGEMENT Chapter 7 - MONITORING AND EVALUATION Chapter 8 - STAKEHOLDERS Chapter 9 - IMPLEMENTATION MECHANISM Chapter 10 - OTHER RELEVANT INFORMATION ANNEXED DOCUMENTS Chapter 1. IDENTIFICATION a - Country: Netherlands b - Name of the area: Bonaire National Marine Park c - Administrative region: Bonaire d - Date of establishment: 1/1/79 e - If different, date of legal declaration: not specified f - Geographic location Longitude X: -68.280058 Latitude Y: 12.134495 g - Size: 27 sq. km h - Contacts Contact adress: STINAPA Bonaire P.O. BOX 368, Bonaire, Dutch Caribbean Headquarter visitor's address: Barcadera z/n, Bonaire, Dutch Caribbean Website: www.bmp.org Email address: [email protected] i - Marine ecoregion 66. Southern Caribbean Comment, optional Chapter 2. EXECUTIVE SUMMARY Present briefly the proposed area and its principal characteristics, and specify the objectives that motivated its creation : The Bonaire National Marine Park was first established in 1979. -

FM), 3-9 July, 3-10 September and 10-13 December 1990
BULLETIN OF THE ALLYN MUSEUM 3621 Bayshore Rd. Sarasota, Florida 34234 Published By Florida Museum of Natural History University of Florida Gainesville, Florida 32611 Number 133 14 June 1991 ISSN-0097-3211 THE BUTTERFLIES OF ANEGADA, BRITISH VIRGIN ISLANDS, WITH DESCRIPTIONS OF A NEW CALISTO (SATYRIDAE) AND A NEW COPAEODES (HESPERIIDAE) ENDEMIC TO THE ISLAND David Spencer Smith Hope Entomological Collections, The University Museum, Parks Road, Oxford, OX! 3PW, England. Lee D. Miller Allyn Museum of Entomology of the Florida Museum of Natural History, 3621 Bay Shore Road, Sarasota, Florida 34234, U.S.A. Faustino KcKenzie Institute of Neurobiology, University of Puerto Rico, Boulevard del Valle 201, Old San Juan, Puerto Rico 00901, U.S.A. This paper is dedicated to the memory of John Griffith of Jesus College, Oxford. INTRODUCTION Anegada island is the northernmost member of the Lesser Antillean arc, situated at 18" 43'N and 64" 19'W. Its nearest neighbors are Anguilla, about 80 statute miles (127 km} across the Anegada Passage to the east-southeast and Virgin Gorda, about 13 miles (21 km} due south. Whereas the Virgin Islands are generally mountainous, Anegada reaches perhaps 18 ' above mean sea level and much of the island is considerably lower (D 'Arcy, 1975}. It is about 10 miles (16 km} in length, about 15 square miles (39 km'} in area, oriented along the east-west axis and is just over 2 miles (3.5 km} across the widest point (Fig. 16}. From the south coast and into the Anegada Passage to the southeast extends the Horseshoe Reef, long a hazard to navigation. -

Food Quality, Competition, and Parasitism Influence Feeding Preference in a Neotropical Lepidopteran
Ecology, 87(12), 2006, pp. 3058–3069 Ó 2006 by the Ecological Society of America FOOD QUALITY, COMPETITION, AND PARASITISM INFLUENCE FEEDING PREFERENCE IN A NEOTROPICAL LEPIDOPTERAN 1,2,3 2 2 1,2 THOMAS A. KURSAR, BRETT T. WOLFE, MARY JANE EPPS, AND PHYLLIS D. COLEY 1Department of Biology, University of Utah, Salt Lake City, Utah 84112 USA 2Smithsonian Tropical Research Institute, Balboa, Panama Abstract. We surveyed Lepidoptera found on 11 species of Inga (Fabaceae:Mimosoideae) co-existing on Barro Colorado Island, Panama, to evaluate factors influencing diet choice. Of the 47 species of caterpillars (747 individuals) recorded, each fed on a distinct set of Inga.In the field, 96% of the individuals were found on young leaves. Growth rates of caterpillars that were fed leaves in the laboratory were 60% higher on young leaves compared to mature leaves. When caterpillars were fed leaves of nonhost Inga, they grew more slowly. These data provide support for a link between preference and performance. However, among hosts on which larvae normally occurred, faster growth rates were not associated with greater host electivity (the proportion of larvae found on each host species in the field, corrected for host abundance). Growth rates on normal hosts were positively correlated with leaf expansion rates of the host, and fast expansion was associated with leaves with higher nutritional content. Detailed studies on a gelechiid leaf roller, the species with the largest diet breadth, allowed us to assess the importance of factors other than growth that could influence diet electivity. This species showed a 1.7-fold difference in growth rate among Inga hosts and faster growth on species with fast-expanding leaves. -

Arthropods of Elm Fork Preserve
Arthropods of Elm Fork Preserve Arthropods are characterized by having jointed limbs and exoskeletons. They include a diverse assortment of creatures: Insects, spiders, crustaceans (crayfish, crabs, pill bugs), centipedes and millipedes among others. Column Headings Scientific Name: The phenomenal diversity of arthropods, creates numerous difficulties in the determination of species. Positive identification is often achieved only by specialists using obscure monographs to ‘key out’ a species by examining microscopic differences in anatomy. For our purposes in this survey of the fauna, classification at a lower level of resolution still yields valuable information. For instance, knowing that ant lions belong to the Family, Myrmeleontidae, allows us to quickly look them up on the Internet and be confident we are not being fooled by a common name that may also apply to some other, unrelated something. With the Family name firmly in hand, we may explore the natural history of ant lions without needing to know exactly which species we are viewing. In some instances identification is only readily available at an even higher ranking such as Class. Millipedes are in the Class Diplopoda. There are many Orders (O) of millipedes and they are not easily differentiated so this entry is best left at the rank of Class. A great deal of taxonomic reorganization has been occurring lately with advances in DNA analysis pointing out underlying connections and differences that were previously unrealized. For this reason, all other rankings aside from Family, Genus and Species have been omitted from the interior of the tables since many of these ranks are in a state of flux. -

Lista De Anexos
LISTA DE ANEXOS ANEXO N°1 MAPA DEL HUMEDAL ANEXO N°2 REGIMEN DE MAREAS SAN JUAN DEL N. ANEXO N°3 LISTA PRELIMINAR DE FAUNA SILVESTRE ANEXO N°4 LISTA PRELIMINAR DE VEGETACIÓN ANEXO N°5 DOSSIER FOTOGRAFICO 22 LISTADO PRELIMINAR DE ESPECIES DE FAUNA SILVESTRE DEL REFUGIO DE VIDA SILVESTRE RIO SAN JUAN. INSECTOS FAMILIA ESPECIE REPORTADO POR BRENTIDAE Brentus anchorago Giuliano Trezzi CERAMBYCIDAE Acrocinus longimanus Giuliano Trezzi COCCINELLIDAE Epilachna sp. Giuliano Trezzi COENAGRIONIDAE Argia pulla Giuliano Trezzi COENAGRIONIDAE Argia sp. Giuliano Trezzi FORMICIDAE Atta sp. Giuliano Trezzi FORMICIDAE Paraponera clavata Giuliano Trezzi FORMICIDAE Camponotus sp. Giuliano Trezzi GOMPHIDAE Aphylla angustifolia Giuliano Trezzi LIBELLULIDAE Micrathyria aequalis Giuliano Trezzi LIBELLULIDAE Micrathyria didyma Giuliano Trezzi LIBELLULIDAE Erythemis peruviana Giuliano Trezzi LIBELLULIDAE Erythrodiplax connata Giuliano Trezzi LIBELLULIDAE Erythrodiplax ochracea Giuliano Trezzi LIBELLULIDAE Dythemis velox Giuliano Trezzi LIBELLULIDAE Idiataphe cubensis Giuliano Trezzi NYMPHALIDAE Caligo atreus Javier Baltodano NYMPHALIDAE Archaeoprepona demophoon Javier Baltodano NYMPHALIDAE Eueides lybia Javier Baltodano NYMPHALIDAE Dryas iulia Javier Baltodano NYMPHALIDAE Heliconius charitonius Javier Baltodano NYMPHALIDAE Heliconius cydno Javier Baltodano NYMPHALIDAE Heliconius erato Javier Baltodano NYMPHALIDAE Heliconius melponeme Javier Baltodano NYMPHALIDAE Heliconius sara Javier Baltodano NYMPHALIDAE Philaetria dido Javier Baltodano NYMPHALIDAE Aeria eurimedia -

Inter-American Convention for the Protection and Conservation of Sea Turtles Caribbean Netherlands Annual Report 2019
Inter-American Convention for the Protection and Conservation of Sea Turtles Caribbean Netherlands Annual Report 2019 IAC Annual Report General Instructions Annex IV of the Convention text states that each Contracting Party shall hand in an Annual Report. To complete this Annual Report, Focal Points should consult with various stakeholders involved in sea turtle issues. If you have any questions regarding this Annual Report, please write to the Secretariat Pro Tempore at [email protected] Please note that the date to submit this Annual Report is April 30th, 2019. Part I (General Information) Please fill out the following tables. Add additional rows if necessary. a._ Focal Point Ministry of Agriculture, Nature and Food Institution Quality of the Netherlands, National Office for Caribbean Netherlands Name Paul Hoetjes Date Annual Report submitted 30 April 2019 b._ Agency or Institution responsible for preparing this report Ministry of Agriculture, Nature and Food Name of Agency or Institution Quality, National Office for the Caribbean Netherlands Name of the person responsible for Paul Hoetjes completing this report Address Kaya Gobernador Debrot 46 Telephone(s) +599 715 8308 Fax [email protected] E-mail Inter-American Convention for the Protection and Conservation of Sea Turtles Country Annual Report 2019 c ._ Others who participated in the preparation of this report Name Agency or Institution E-mail Mabel Nava Sea Turtle Conservation Bonaire [email protected] Jessica Berkel St. Eustatius National Parks [email protected] Foundation Part II (Policy and Management) a._ General description of activities carried out for the protection and conservation of sea turtles In accordance with Articles IX and XVIII of the text of the Convention, each Party shall establish monitoring programs, policies and plans for implementation at a national level for the protection and conservation of sea turtles and their habitat. -

Nature Policy Plan the Caribbean Netherlands
Nature Policy Plan The Caribbean Netherlands Nature Policy for the Caribbean Netherlands 2013-2017 Nature Policy Plan The Caribbean Netherlands 2013 - 2017 Contents A | Introduction A Introduction 3 Aruba, Curaçao, St. Maarten, Bonaire, Saba and St. Eustatius Process 4 Policy Objective and Function 4 form the Dutch Caribbean within the Kingdom of the Netherlands. The Kingdom of the Netherlands is a B The Caribbean Netherlands in Context 8 1 Nature and Biodiversity 8 comprehensive sovereign state made up of four countries 2 Threats 8 3 Nature as an Economic Resource 10 of which the Netherlands is one. Aruba, Curaçao, and 4 Legal Framework 10 St. Maarten each form one of the three remaining constituent C Roles and responsibilities 14 countries, while the other islands, Bonaire, St. Eustatius, and 1 National Government 15 2 The Island Governments 15 Saba, are Dutch overseas public bodies and as such are part 3 Non-Governmental Nature Conservation Organisations (NGOs) 16 4 International Cooperation 16 of the country of the Netherlands. Collectively these three islands are known as the Caribbean Netherlands and are the D Resources 20 1 National Governement 20 focus of the present Nature Policy Plan. Where possible, 2 Local 21 3 Donations 22 this Nature Policy Plan will be implemented in line with the E Strategy and goals 24 Nature Policy Plans of the other constituent countries of 1 Mainstreaming 24 2 Nature Management 24 the Kingdom. 3 Strategic goals and actions 33 The Dutch Caribbean islands show great biological diversity and support hundreds of endemic species and ecosystems some of which are globally threatened.