Oxygen Consumption of the Gag Grouper
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Honoring the Family Meal Helping Families Be Healthier and Happier with Quick, Easy Seafood Meals
Honoring the Family Meal Helping families be healthier and happier with quick, easy seafood meals OCTOBER IS NATIONAL SEAFOOD MONTH Seafood Nutrition Partnership is here to inspire Americans to enjoy seafood at least twice a week by showing how buying and Fun ways to preparing seafood is simple and delicious! USE THIS TOOLKIT For National Seafood Month in October, we are bringing the This toolkit is designed to inspire and focus back to family. demonstrate how you can implement National Seafood Month this October. Magic happens during family mealtime when children and parents Within the theme, several messaging tracks gather around the table and engage each other in conversation. Family meals eaten at home have been proven to benefit the will be promoted to speak to different health and wellness of children and adolescents, to fight obesity consumers. Please utilize the turnkey and substance abuse, and to make families stronger—creating a resources and messaging, or work with us to positive impact on our communities and our nation as a whole. customize it for your audiences. Join us as we work collaboratively with health partners, ▶ Quick, Easy Weeknight Meals seafood companies, retailers and dietitians from across the Many fish dishes cook in 15 minutes or less country to bring families back to the table. ▶ Fun Ways to Engage Seafood Nutrition Partnership is partnering with the Food Little Seafoodies Marketing Institute Foundation to emphasize the importance of Get kids cooking in the kitchen family meals, expanding National Family Meals Month throughout the year and into a true movement — the Family Meals Movement. -

Term Respiratory Quotient (RQ) of Wastewater Samples
International Research Journal of Engineering and Technology (IRJET) e-ISSN: 2395-0056 Volume: 05 Issue: 10 | Oct 2018 www.irjet.net p-ISSN: 2395-0072 Design and Fabrication of a Micro-respirometer to Measure the Short- Term Respiratory Quotient (RQ) of Wastewater Samples M. S. Rahman1, M. Z. Mousumi2, M. A. A. Sakib2, M. S. A. Fahad2, T. J. CHY2 & N. M. H. Robiul3 1Associate Professor, Department of Civil and Environmental Engineering, Shahjalal University of Science and Technology, Sylhet, Bangladesh. 2Student, Department of Civil and Environmental Engineering, Shahjalal University of Science and Technology, Sylhet, Bangladesh. 3Assistant Professor, Department of Civil and Environmental Engineering, Shahjalal University of Science and Technology, Sylhet, Bangladesh. ----------------------------------------------------------------------***--------------------------------------------------------------------- Abstract - The main objective of the study is to develop a activity [4, 5]. Therefore, the respiration process and its low-cost wastewater micro-respirometer to determine some qualitative and quantitative phenomena could be an effective important bioactivity performance indicators such as oxygen indicator of the on-going condition of a typical consumption rate (OCR), carbon dioxide evolution rate (CER) biodegradation process. Respirometric parameters such as and respiratory quotient (RQ) of wastewater samples collected cumulative oxygen consumption (COC), oxygen consumption from effluent treatment plants (ETPs) and other wastewater rate (OCR), cumulative carbon-dioxide evolution (CCE), sources. The developed apparatus was comprised of two major carbon dioxide evolution rate (CER) and respiratory parts, a reaction chamber and a measuring unit facilitated quotient (RQ) can ascertain the presence of organic matters, with a well type vertical volumeter. Validation of the newly microbes and respiration type present in wastewater by fabricated respirometer was done by checking the observing microbial activities [4, 5, 6]. -

Monitoring Anesthetic Depth
ANESTHETIC MONITORING Lyon Lee DVM PhD DACVA MONITORING ANESTHETIC DEPTH • The central nervous system is progressively depressed under general anesthesia. • Different stages of anesthesia will accompany different physiological reflexes and responses (see table below, Guedel’s signs and stages). Table 1. Guedel’s (1937) Signs and Stages of Anesthesia based on ‘Ether’ anesthesia in cats. Stages Description 1 Inducement, excitement, pupils constricted, voluntary struggling Obtunded reflexes, pupil diameters start to dilate, still excited, 2 involuntary struggling 3 Planes There are three planes- light, medium, and deep More decreased reflexes, pupils constricted, brisk palpebral reflex, Light corneal reflex, absence of swallowing reflex, lacrimation still present, no involuntary muscle movement. Ideal plane for most invasive procedures, pupils dilated, loss of pain, Medium loss of palpebral reflex, corneal reflexes present. Respiratory depression, severe muscle relaxation, bradycardia, no Deep (early overdose) reflexes (palpebral, corneal), pupils dilated Very deep anesthesia. Respiration ceases, cardiovascular function 4 depresses and death ensues immediately. • Due to arrival of newer inhalation anesthetics and concurrent use of injectable anesthetics and neuromuscular blockers the above classic signs do not fit well in most circumstances. • Modern concept has two stages simply dividing it into ‘awake’ and ‘unconscious’. • One should recognize and familiarize the reflexes with different physiologic signs to avoid any untoward side effects and complications • The system must be continuously monitored, and not neglected in favor of other signs of anesthesia. • Take all the information into account, not just one sign of anesthetic depth. • A major problem faced by all anesthetists is to avoid both ‘too light’ anesthesia with the risk of sudden violent movement and the dangerous ‘too deep’ anesthesia stage. -
Clean &Unclean Meats
Clean & Unclean Meats God expects all who desire to have a relationship with Him to live holy lives (Exodus 19:6; 1 Peter 1:15). The Bible says following God’s instructions regarding the meat we eat is one aspect of living a holy life (Leviticus 11:44-47). Modern research indicates that there are health benets to eating only the meat of animals approved by God and avoiding those He labels as unclean. Here is a summation of the clean (acceptable to eat) and unclean (not acceptable to eat) animals found in Leviticus 11 and Deuteronomy 14. For further explanation, see the LifeHopeandTruth.com article “Clean and Unclean Animals.” BIRDS CLEAN (Eggs of these birds are also clean) Chicken Prairie chicken Dove Ptarmigan Duck Quail Goose Sage grouse (sagehen) Grouse Sparrow (and all other Guinea fowl songbirds; but not those of Partridge the corvid family) Peafowl (peacock) Swan (the KJV translation of “swan” is a mistranslation) Pheasant Teal Pigeon Turkey BIRDS UNCLEAN Leviticus 11:13-19 (Eggs of these birds are also unclean) All birds of prey Cormorant (raptors) including: Crane Buzzard Crow (and all Condor other corvids) Eagle Cuckoo Ostrich Falcon Egret Parrot Kite Flamingo Pelican Hawk Glede Penguin Osprey Grosbeak Plover Owl Gull Raven Vulture Heron Roadrunner Lapwing Stork Other birds including: Loon Swallow Albatross Magpie Swi Bat Martin Water hen Bittern Ossifrage Woodpecker ANIMALS CLEAN Leviticus 11:3; Deuteronomy 14:4-6 (Milk from these animals is also clean) Addax Hart Antelope Hartebeest Beef (meat of domestic cattle) Hirola chews -

Fish Species of Saskatchewan
Introduction From the shallow, nutrient -rich potholes of the prairies to the clear, cool rock -lined waters of our province’s north, Saskatchewan can boast over 50,000 fish-bearing bodies of water. Indeed, water accounts for about one-eighth, or 80,000 square kilometers, of this province’s total surface area. As numerous and varied as these waterbodies are, so too are the types of fish that inhabit them. In total, Saskatchewan is home to 67 different fish species from 16 separate taxonomic families. Of these 67, 58 are native to Saskatchewan while the remaining nine represent species that have either been introduced to our waters or have naturally extended their range into the province. Approximately one-third of the fish species found within Saskatchewan can be classed as sportfish. These are the fish commonly sought out by anglers and are the best known. The remaining two-thirds can be grouped as minnow or rough-fish species. The focus of this booklet is primarily on the sportfish of Saskatchewan, but it also includes information about several rough-fish species as well. Descriptions provide information regarding the appearance of particular fish as well as habitat preferences and spawning and feeding behaviours. The individual species range maps are subject to change due to natural range extensions and recessions or because of changes in fisheries management. "...I shall stay him no longer than to wish him a rainy evening to read this following Discourse; and that, if he be an honest Angler, the east wind may never blow when he goes a -fishing." The Compleat Angler Izaak Walton, 1593-1683 This booklet was originally published by the Saskatchewan Watershed Authority with funds generated from the sale of angling licences and made available through the FISH AND WILDLIFE DEVELOPMENT FUND. -

Northern Pike Esox Lucius ILLINOIS RANGE
northern pike Esox lucius Kingdom: Animalia FEATURES Phylum: Chordata The northern pike's average life span is eight to 10 Class: Osteichthyes years. The average weight is two pounds. It may Order: Esociformes attain a maximum length of 53 inches. The fins are rounded, and all except the pectoral fins have dark Family: Esocidae spots. Scales are present on the cheek and half of ILLINOIS STATUS the gill cover. The eyes are yellow. The long, green body has yellow spots on the sides. The belly is common, native white to dark yellow. The duckbill-shaped snout is easily seen. BEHAVIORS The northern pike lives in lakes, rivers and marshes. It prefers water without strong currents and with many plants. This fish reaches maturity at age two to three years. Spawning occurs in March. The female deposits up to 150,000 eggs that are scattered in marshy areas or other shallow water areas. Eggs hatch in 12-14 days. This fish eats fishes, insects, crayfish, frogs and reptiles. ILLINOIS RANGE © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. close up of head close up of side © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources. © Engbretson Underwater Photography adult Aquatic Habitats lakes, ponds and reservoirs; rivers and streams; marshes Woodland Habitats none Prairie and Edge Habitats none © Illinois Department of Natural Resources. 2020. Biodiversity of Illinois. Unless otherwise noted, photos and images © Illinois Department of Natural Resources.. -

Fisheries Full Issue PDF Volume 39, Issue 3
This article was downloaded by: [American Fisheries Society] On: 24 March 2014, At: 13:30 Publisher: Taylor & Francis Informa Ltd Registered in England and Wales Registered Number: 1072954 Registered office: Mortimer House, 37-41 Mortimer Street, London W1T 3JH, UK Fisheries Publication details, including instructions for authors and subscription information: http://www.tandfonline.com/loi/ufsh20 Full Issue PDF Volume 39, Issue 3 Published online: 21 Mar 2014. To cite this article: (2014) Full Issue PDF Volume 39, Issue 3, Fisheries, 39:3, 97-144, DOI: 10.1080/03632415.2014.902705 To link to this article: http://dx.doi.org/10.1080/03632415.2014.902705 PLEASE SCROLL DOWN FOR ARTICLE Taylor & Francis makes every effort to ensure the accuracy of all the information (the “Content”) contained in the publications on our platform. However, Taylor & Francis, our agents, and our licensors make no representations or warranties whatsoever as to the accuracy, completeness, or suitability for any purpose of the Content. Any opinions and views expressed in this publication are the opinions and views of the authors, and are not the views of or endorsed by Taylor & Francis. The accuracy of the Content should not be relied upon and should be independently verified with primary sources of information. Taylor and Francis shall not be liable for any losses, actions, claims, proceedings, demands, costs, expenses, damages, and other liabilities whatsoever or howsoever caused arising directly or indirectly in connection with, in relation to or arising out of the use of the Content. This article may be used for research, teaching, and private study purposes. -

APPENDIX M Common and Scientific Species Names
Bay du Nord Development Project Environmental Impact Statement APPENDIX M Common and Scientific Species Names Bay du Nord Development Project Environmental Impact Statement Common and Species Names Common Name Scientific Name Fish Abyssal Skate Bathyraja abyssicola Acadian Redfish Sebastes fasciatus Albacore Tuna Thunnus alalunga Alewife (or Gaspereau) Alosa pseudoharengus Alfonsino Beryx decadactylus American Eel Anguilla rostrata American Plaice Hippoglossoides platessoides American Shad Alosa sapidissima Anchovy Engraulidae (F) Arctic Char (or Charr) Salvelinus alpinus Arctic Cod Boreogadus saida Atlantic Bluefin Tuna Thunnus thynnus Atlantic Cod Gadus morhua Atlantic Halibut Hippoglossus hippoglossus Atlantic Mackerel Scomber scombrus Atlantic Salmon (landlocked: Ouananiche) Salmo salar Atlantic Saury Scomberesox saurus Atlantic Silverside Menidia menidia Atlantic Sturgeon Acipenser oxyrhynchus oxyrhynchus Atlantic Wreckfish Polyprion americanus Barndoor Skate Dipturus laevis Basking Shark Cetorhinus maximus Bigeye Tuna Thunnus obesus Black Dogfish Centroscyllium fabricii Blue Hake Antimora rostrata Blue Marlin Makaira nigricans Blue Runner Caranx crysos Blue Shark Prionace glauca Blueback Herring Alosa aestivalis Boa Dragonfish Stomias boa ferox Brook Trout Salvelinus fontinalis Brown Bullhead Catfish Ameiurus nebulosus Burbot Lota lota Capelin Mallotus villosus Cardinal Fish Apogonidae (F) Chain Pickerel Esox niger Common Grenadier Nezumia bairdii Common Lumpfish Cyclopterus lumpus Common Thresher Shark Alopias vulpinus Crucian Carp -

Esox Lucius) Ecological Risk Screening Summary
Northern Pike (Esox lucius) Ecological Risk Screening Summary U.S. Fish & Wildlife Service, February 2019 Web Version, 8/26/2019 Photo: Ryan Hagerty/USFWS. Public Domain – Government Work. Available: https://digitalmedia.fws.gov/digital/collection/natdiglib/id/26990/rec/22. (February 1, 2019). 1 Native Range and Status in the United States Native Range From Froese and Pauly (2019a): “Circumpolar in fresh water. North America: Atlantic, Arctic, Pacific, Great Lakes, and Mississippi River basins from Labrador to Alaska and south to Pennsylvania and Nebraska, USA [Page and Burr 2011]. Eurasia: Caspian, Black, Baltic, White, Barents, Arctic, North and Aral Seas and Atlantic basins, southwest to Adour drainage; Mediterranean basin in Rhône drainage and northern Italy. Widely distributed in central Asia and Siberia easward [sic] to Anadyr drainage (Bering Sea basin). Historically absent from Iberian Peninsula, Mediterranean France, central Italy, southern and western Greece, eastern Adriatic basin, Iceland, western Norway and northern Scotland.” Froese and Pauly (2019a) list Esox lucius as native in Armenia, Azerbaijan, China, Georgia, Iran, Kazakhstan, Mongolia, Turkey, Turkmenistan, Uzbekistan, Albania, Austria, Belgium, Bosnia Herzegovina, Bulgaria, Croatia, Czech Republic, Denmark, Estonia, Finland, France, Germany, Greece, Hungary, Ireland, Italy, Latvia, Lithuania, Luxembourg, Macedonia, Moldova, Monaco, 1 Netherlands, Norway, Poland, Romania, Russia, Serbia, Slovakia, Slovenia, Sweden, Switzerland, United Kingdom, Ukraine, Canada, and the United States (including Alaska). From Froese and Pauly (2019a): “Occurs in Erqishi river and Ulungur lake [in China].” “Known from the Selenge drainage [in Mongolia] [Kottelat 2006].” “[In Turkey:] Known from the European Black Sea watersheds, Anatolian Black Sea watersheds, Central and Western Anatolian lake watersheds, and Gulf watersheds (Firat Nehri, Dicle Nehri). -

The Use of Oxygen Consumption and Ammonium Excretion To
Chemosphere 84 (2011) 9–16 Contents lists available at ScienceDirect Chemosphere journal homepage: www.elsevier.com/locate/chemosphere The use of oxygen consumption and ammonium excretion to evaluate the toxicity of cadmium on Farfantepenaeus paulensis with respect to salinity ⇑ Edison Barbieri a, , Eduardo Tavares Paes b a Instituto de Pesca – APTA – SAA/SP, Caixa Postal 61, Cananéia 11990-000, São Paulo, Brazil b Instituto Socioambiental e dos Recursos Hídricos (ISARH) – Universidade Federal Rural da Amazônia (UFRA), Brazil article info abstract Article history: The main purpose of the present study was to detect the acute toxicity of cadmium (Cd) in F. paulensis Received 1 November 2010 and to investigate its effect on oxygen consumption and ammonium excretion different salinities. First, Received in revised form 8 February 2011 we examined the acute toxicity of Cd in F. paulensis at 24, 48, 72, and 96-h lethal concentration Accepted 14 February 2011 (LC50). Cd was significantly more toxic at 5 salinity than at 20 and 36. The oxygen consumption and Available online 7 April 2011 ammonium excretion were estimated through experiments performed on each of the twelve possible combinations of three salinities (36, 20 and 5), at temperature 20 °C. Cd showed a reduction in oxygen Keywords: consumption at 5 salinity, the results show that the oxygen consumption decreases with respect to Shrimp the Cd concentration. At the highest Cd concentration employed (2 mg LÀ1), the salinity 5 and the tem- Cd Salinity perature at 20 °C, oxygen consumption decreases 53.7% in relation to the control. In addition, after sep- Oxygen consumption arate exposure to Cd, elevation in ammonium excretion was obtained, wish were 72%, 65% and 95% Ammonium excretion higher than the control, respectively. -
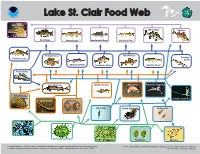
Lake St. Clair Food Web MENT of C
ATMOSPH ND ER A I C C I A N D A M E I C N O I S L T A R N A T O I I O T N A N U E .S C .D R E E PA M RT OM Lake St. Clair Food Web MENT OF C Sea Lamprey White Bass Walleye Rock Bass Muskellunge Smallmouth Bass Northern Pike Pumpkinseed Yellow Perch Rainbow Common Carp Smelt Channel Catfish Freshwater Drum Spottail Shiner Round Goby Lake Sturgeon Mayfly nymphs Gammarus Raptorial waterflea Zebra/Quagga mussels Invasive waterflea Chironomids Mollusks Calanoids Native waterflea Cyclopoids Diatoms Green algae Blue-green algae Flagellates Rotifers Foodweb based on “Impact of exotic invertebrate invaders on food web structure and function in the Great Lakes: NOAA, Great Lakes Environmental Research Laboratory, 4840 S. State Road, Ann Arbor, MI A network analysis approach” by Mason, Krause, and Ulanowicz, 2002 - Modifications for Lake St. Clair, 2009. 734-741-2235 - www.glerl.noaa.gov Lake St. Clair Food Web Sea Lamprey Planktivores/Benthivores Sea lamprey (Petromyzon marinus). An aggressive, non-native parasite that Lake Sturgeon (Acipenser fulvscens). Endangered over most of its historic fastens onto its prey and rasps out a hole with its rough tongue. range. Its diet commonly includes small clams, snails, crayfish, sideswimmers, aquatic insect larvae, algae, and other plant matter. Piscivores (Fish Eaters) Macroinvertebrates White bass (Morone Chrysops). Prefers clear open water in lakes and large rivers. Chironomids/Oligochaetes. Larval insects and worms that live on the lake Visual feeders, uses sight instead of smell to find prey. -

Current Ocean Wise Approved Canadian MSC Fisheries
Current Ocean Wise approved Canadian MSC Fisheries Updated: November 14, 2017 Legend: Blue - Ocean Wise Red - Not Ocean Wise White - Only specific areas or gear types are Ocean Wise Species Common Name Latin Name MSC Fishery Name Gear Location Reason for Exception Clam Clearwater Seafoods Banquereau and Banquereau Bank Artic surf clam Mactromeris polynyma Grand Banks Arctic surf clam Hydraulic dredges Grand Banks Crab Snow Crab Chionoecetes opilio Gulf of St Lawrence snow crab trap Conical or rectangular crab pots (traps) North West Atlantic - Nova Scotia Snow Crab Chionoecetes opilio Scotian shelf snow crab trap Conical or rectangular crab pots (traps) North West Atlantic - Nova Scotia Snow Crab Chionoecetes opilio Newfoundland & Labrador snow crab Pots Newfoundland & Labrador Flounder/Sole Yellowtail flounder Limanda ferruginea OCI Grand Bank yellowtail flounder trawl Demersal trawl Grand Banks Haddock Trawl Bottom longline Gillnet Hook and Line CAN - Scotian shelf 4X5Y Trawl Bottom longline Gillnet Atlantic haddock Melangrammus aeglefinus Canada Scotia-Fundy haddock Hook and Line CAN - Scotian shelf 5Zjm Hake Washington, Oregon and California North Pacific hake Merluccius productus Pacific hake mid-water trawl Mid-water Trawl British Columbia Halibut Pacific Halibut Hippoglossus stenolepis Canada Pacific halibut (British Columbia) Bottom longline British Columbia Longline Nova Scotia and Newfoundland Gillnet including part of the Grand banks and Trawl Georges bank, NAFO areas 3NOPS, Atlantic Halibut Hippoglossus hippoglossus Canada