Molecular Biology of Cancer: Mechanisms, Targets
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

2004 Albert Lasker Nomination Form
albert and mary lasker foundation 110 East 42nd Street Suite 1300 New York, ny 10017 November 3, 2003 tel 212 286-0222 fax 212 286-0924 Greetings: www.laskerfoundation.org james w. fordyce On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination Chairman neen hunt, ed.d. for the 2004 Albert Lasker Medical Research Awards. President mrs. anne b. fordyce The Awards will be offered in three categories: Basic Medical Research, Clinical Medical Vice President Research, and Special Achievement in Medical Science. This is the 59th year of these christopher w. brody Treasurer awards. Since the program was first established in 1944, 68 Lasker Laureates have later w. michael brown Secretary won Nobel Prizes. Additional information on previous Lasker Laureates can be found jordan u. gutterman, m.d. online at our web site http://www.laskerfoundation.org. Representative Albert Lasker Medical Research Awards Program Nominations that have been made in previous years may be updated and resubmitted in purnell w. choppin, m.d. accordance with the instructions on page 2 of this nomination booklet. daniel e. koshland, jr., ph.d. mrs. william mccormick blair, jr. the honorable mark o. hatfied Nominations should be received by the Foundation no later than February 2, 2004. Directors Emeritus A distinguished panel of jurors will select the scientists to be honored. The 2004 Albert Lasker Medical Research Awards will be presented at a luncheon ceremony given by the Foundation in New York City on Friday, October 1, 2004. Sincerely, Joseph L. Goldstein, M.D. Chairman, Awards Jury Albert Lasker Medical Research Awards ALBERT LASKER MEDICAL2004 RESEARCH AWARDS PURPOSE AND DESCRIPTION OF THE AWARDS The major purpose of these Awards is to recognize and honor individuals who have made signifi- cant contributions in basic or clinical research in diseases that are the main cause of death and disability. -

In Vivo Kinetics of Thymidylate Synthetase Inhibition in 5-Fluorouracil- Sensitive and -Resistant Murine Colon Adenocarcinomas1
[CANCER RESEARCH 42, 450-456, February 1982) 0008-5472/82/0042-OOOOS02.00 In Vivo Kinetics of Thymidylate Synthetase Inhibition in 5-Fluorouracil- sensitive and -resistant Murine Colon Adenocarcinomas1 C. Paul Spears, Antranik H. Shahinian, Richard G. Moran, Charles Heidelberger,2 and Thomas H. Corbett Cancer Research Laboratories, University of Southern California Comprehensive Cancer Center, Los Angeles, California 90033 1C. P. S., A. H. S., C. H.]; Childrens Hospital of Los Angeles, Los Angeles, California 9002 7 [R. G. M.I and Southern Research Institute, Birmingham, Alabama 35255 [T. H. C.] ABSTRACT is inactivated rapidly in the presence of the 5-FUra metabolite, FdUMP, and CH2FH4, by the formation of an enzyme:FdUMP: The predictive utility of several biochemical parameters of 5- CH2FH4 covalently bonded ternary complex (10, 25, 43), from fluorouracil (5-FUra) action was evaluated in four murine co- which native TS is slowly released (11, 29, 48). Ionic adenocarcinomas: 5-FUra-sensitive Tumor 38 and 5- Evidence that TS inactivation is the critical therapeutic effect FUra-resistant Tumors 07/A, 51, and 06/A. Thymidylate syn- of 5-FUra has been largely indirect, because of difficulties in thetase (TS) was determined by a tritiated 5-fluoro-2'-deoxyu- the assay of low, growth-limiting levels of TS. Recent studies ridylate (FdUMP)-binding assay. Bolus 5-FUra (80 mg/kg, i.p.) using a sensitive tritium release TS assay (42), however, have administration caused in all tumors a rapid decrease in free TS indicated correlations between 5-FUra cytotoxicity and de levels. Only Tumor 38, however, showed inhibition of TS to creases in tumor TS activity (3, 12). -

Optimal Scheduling of Methotrexate and 5-Fluorouracil in Human Breast Cancer1
[CANCER RESEARCH 42, 2081-2086, May 1982] 0008-5472/82/0042-0000$02.00 Optimal Scheduling of Methotrexate and 5-Fluorouracil in Human Breast Cancer1 Chris Benz, Tina Tillis, Ellen Tattelman, and Ed Cadman2 Departments of Medicine and Pharmacology, Yale School of Medicine, New Haven, Connecticut 06510 ABSTRACT tually die of disseminated disease (11, 25). The use of combi nation chemotherapy in disseminated breast cancer has im We have shown previously that methotrexate pretreatment proved objective response rates over single-agent therapy by of murine leukemia and human colon carcinoma cell cultures about 40%, yet there has been no improvement in overall results in augmented intracellular accumulation of 5-fluorour- survival and no clear superiority in palliative benefit over the acil metabolites. Both of these drugs are commonly used for use of sequential single-agent therapy (9, 18). These discour the treatment of women with breast cancer; thus, sequencing aging facts reflect the brevity of response durations following of methotrexate before 5-fluorouracil was evaluated in vitro systemic chemotherapy, 7 to 11 months, and low complete using a human mammary carcinoma cell line, 47-DN. Intracel response rates of about 15% (7). Perhaps more knowledgeable lular 5-fluorouracil accumulation was maximally increased 4- scheduling of multiple drugs given in combination will improve fold in cultures pretreated with 10 /ÕMmethotrexate for 24 hr. our therapeutic impact on breast cancer; basic laboratory This enhancement of 5-fluorouracil metabolism was associated studies may be able to provide the necessary rationale for with increased intracellular levels of 5-phosphoribosyl 1-pyro- devising such synergistic combinations. -

Lasker Interactive Research Nom'18.Indd
THE 2018 LASKER MEDICAL RESEARCH AWARDS Nomination Packet albert and mary lasker foundation November 1, 2017 Greetings: On behalf of the Albert and Mary Lasker Foundation, I invite you to submit a nomination for the 2018 Lasker Medical Research Awards. Since 1945, the Lasker Awards have recognized the contributions of scientists, physicians, and public citizens who have made major advances in the understanding, diagnosis, treatment, cure, and prevention of disease. The Medical Research Awards will be offered in three categories in 2018: Basic Research, Clinical Research, and Special Achievement. The Lasker Foundation seeks nominations of outstanding scientists; nominations of women and minorities are encouraged. Nominations that have been made in previous years are not automatically reconsidered. Please see the Nomination Requirements section of this booklet for instructions on updating and resubmitting a nomination. The Foundation accepts electronic submissions. For information on submitting an electronic nomination, please visit www.laskerfoundation.org. Lasker Awards often presage future recognition of the Nobel committee, and they have become known popularly as “America’s Nobels.” Eighty-seven Lasker laureates have received the Nobel Prize, including 40 in the last three decades. Additional information on the Awards Program and on Lasker laureates can be found on our website, www.laskerfoundation.org. A distinguished panel of jurors will select the scientists to be honored with Lasker Medical Research Awards. The 2018 Awards will -

Enzymes: the Biological Accelerators
Organic and Medicinal Chemistry International Journal ISSN 2474-7610 Review Article Organic & Medicinal Chem IJ Volume 2 Issue 4 - May 2017 Copyright © All rights are reserved by Ravindra K. Rawal DOI: 10.19080/OMCIJ.2016.01.555594 Enzymes: The Biological Accelerators Sundeep Kaur Manjal, Ramandeep Kaur, Rohit Bhatia and Ravindra K. Rawal* Department of Pharmaceutical Chemistry, ISF College of Pharmacy, India Submission: April 06, 2017; Published: May 26,2017 *Corresponding author: Ravindra K. Rawal, Department of Pharmaceutical Chemistry, ISF College of Pharmacy, GT Road Moga, Punjab 142001, India, Tel: ; Email: Abstract Enzymes are the macromolecular biological catalysts which tend to exhibit tremendous biological value for the human society. These are known to accelerate and catalyze the chemical reactions many times faster than ordinary. Generally, they are known to catalyze more than 5,000 types of biochemical reactions. Numerous enzymes are produced inside the human body which tend to play crucial role in the functioning of biological activities. Some of the enzymes are used commercially such as for the synthesis of antibiotics and also used for household purpose like in manufacturing of washing powder. Apart from this, enzymes serve a variety of functions inside the living organisms. Recently pro-drug approach has gained wide popularity, it is mainly utilised in the lead optimization of the drug molecule. In this review we have tried to discuss different aspects of enzymes, enzyme kinetics, enzyme inhibitors, pro-drugs and their utility with suitable examples. Keywords: Enzyme; Serine; Trypsinogen; Heme; Pro-drug Introduction function are determined by four structural features that are - the Enzymes act as catalysts for almost all of the chemical metal core, the metal binding motif, the second sphere residues reactions that occur in all living organisms [1]. -
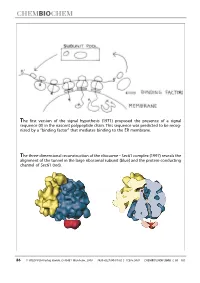
The First Version of the Signal Hypothesis (1971) Proposed the Presence of a Signal Sequence (X) in the Nascent Polypeptide Chain
The first version of the signal hypothesis (1971) proposed the presence of a signal sequence (X) in the nascent polypeptide chain. This sequence was predicted to be recog- nized by a ªbinding factorº that mediates binding to the ER membrane. The three-dimensional reconstruction of the ribosome ± Sec61 complex (1997) reveals the alignment of the tunnel in the large ribosomal subunit (blue) and the protein-conducting channel of Sec61 (red). 86 WILEY-VCH-Verlag GmbH, D-69451 Weinheim, 2000 1439-4227/00/01/02 $ 17.50+.50/0 CHEMBIOCHEM 2000,1,86±102 Protein Targeting (Nobel Lecture)** Günter Blobel*[a] KEYWORDS: membranes ´ Nobel lecture ´ proteins ´ protein transport Prologue I began research in the sixties, first as a graduate student in the laboratory of Van R. Potter (Figure 1) at the McArdle Institute for Cancer Research of the University of Wisconsin in Madison. I continued as a postdoctoral fellow in the laboratory of George E. Palade (Figure 2) at The Rockefeller Uni- versity in New York City. At that time, the intracellular pathway of secretory pro- teins, from their synthesis to their extru- sion from the cell, the so-called ªsecre- tory pathwayº, had already been worked out by George Palade and his co-workers, primarily Philip Siekevitz, Jim Jamieson, and Lucien Caro (for review see the 1974 Nobel lecture by George Palade[1] ). Using Figure 3. The secretory pathway. Secretory proteins (indicated in red) are pulse ± chase labeling with radioactive synthesized on ribosomes bound to the endoplasmic reticulum (ER). They are then amino acids in tissue slices in conjunction transported via vesicular carriers through the Golgi complex and are finally exocytosed. -

Thymidylate Synthetase Overproduction in 5-Fluorodeoxyuridine-Resistant Mouse Fibroblasts CINDY ROSSANA, LAKSHMI GOLLAKOTA RAO, and LEE F
MOLECULAR AND CELLULAR BIOLOGY, Sept. 1982, p. 1118-1125 Vol. 2, No. 9 0270-7306/82/091118-08$02.00/0 Copyright 0 1982, American Society for Microbiology Thymidylate Synthetase Overproduction in 5-Fluorodeoxyuridine-Resistant Mouse Fibroblasts CINDY ROSSANA, LAKSHMI GOLLAKOTA RAO, AND LEE F. JOHNSON* Department ofBiochemistry, The Ohio State University, Columbus, Ohio 43210 Received 9 February 1982/Accepted 29 April 1982 We describe the isolation and characterization of a series of 5-fluorodeoxyuri- dine (FdUrd)-resistant mouse 3T6 cell lines that overproduce thymidylate synthe- tase (TS) by up to 50-fold compared with the parental cells. The resistant cells were selected by growing 3T6 cells or a methotrexate-resistant 3T6 cell line (M50L3, isolated previously in our laboratory) in gradua$ increasing concentra- tions of FdUrd. Uridine and cytidine were included in the culture medium to reduce toxicity from metabolic products of FdUrd. Cells that were resistant to the drug by virtue of loss of thymidine kinase activity were eliminated by selection in medium containing hypoxanthine, methotrexate, and thymidine. M5OL3 cells were found to adapt to FdUrd more readily than 3T6 cells. A number of clones were isolated that were able to grow in the presence of 3 ,uM (M50L3 derived) or 0.3 ,uM (3T6 derived) FdUrd. Several were found to overproduce TS by 10 to 50- fold compared with normal 3T6 cells. All were found to have thymidine kinase activity, although the enzyme level was significantly reduced in some clones. The overproduced TS was inactivated by 5-fluorodeoxyuridylic acid at the same concentration as the enzyme from 3T6 cells. -

5-Fluorouracil: Mechanisms of Action and Clinical Strategies
REVIEWS 5-FLUOROURACIL: MECHANISMS OF ACTION AND CLINICAL STRATEGIES Daniel B. Longley, D. Paul Harkin and Patrick G. Johnston 5-Fluorouracil (5-FU) is widely used in the treatment of cancer. Over the past 20 years, increased understanding of the mechanism of action of 5-FU has led to the development of strategies that increase its anticancer activity. Despite these advances, drug resistance remains a significant limitation to the clinical use of 5-FU. Emerging technologies, such as DNA microarray profiling, have the potential to identify novel genes that are involved in mediating resistance to 5-FU. Such target genes might prove to be therapeutically valuable as new targets for chemotherapy, or as predictive biomarkers of response to 5-FU-based chemotherapy. FLUOROPYRIMIDINES Antimetabolite drugs work by inhibiting essential Understanding the mechanisms by which 5-FU Antimetabolite drugs such as biosynthetic processes, or by being incorporated into causes cell death and by which tumours become resistant 5-fluorouracil that are fluorinated macromolecules, such as DNA and RNA, and inhibiting to 5-FU is an essential step towards predicting or over- derivatives of pyrimidines. their normal function. The fluoropyrimidine 5-fluo- coming that resistance. So, what do we know about the IRINOTECAN rouracil (5-FU) does both. FLUOROPYRIMIDINES were devel- mechanism of action of 5-FU and what strategies have An anticancer drug that inhibits oped in the 1950s following the observation that rat been used to enhance its activity? DNA MICROARRAY technol- DNA topoisomerase I. It is used hepatomas used the pyrimidine uracil — one of the ogy has the potential to identify novel genes that have key in the treatment of advanced four bases found in RNA — more rapidly than normal roles in mediating resistance to 5-FU-based chemother- colorectal cancer. -

Thymidylate Synthase and Drug Resistance
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by DSpace at VU EuropemJ~lofCanVol. %A, Nos. 74 pp. 1299-1305,1995 Copyright 0 1995 Elsevier Science Ltd Pergamon PrintedinGreatBritnin.AUrightsresenred 095wl049/95s9.5o+o.oo Thymidylate Synthase and Drug Resistance G.J. Peters, C.L. van der Wilt, B. van Triest, G. Codacci-Pisanelli, P.G. Johnston, C.J. van Groeningen and H.M. Pinedo Thymidylate synthase is an important target for both fluorinated pyrimidines and for new folate analogues. Resistance to S-fluorouracii (SFU) can be related to insufficient inhibition of thymidylate synthase. The 5FU- nucleotide FdUMP induces inhibition of thymidylate synthase which is enhanced and retained for longer in the presence of increased folate pools, for which leucovorin is a precursor. In a murine model system, 5FU treatment caused a 4-fold induction of thymidylate synthase levels which may have contributed to resistance. Addition of leucovorin to this treatment prevented this induction and increased the antitumour effect 2-3-fold. In the clinical setting, 5FU administration to patients resulted in approximately 50% inhibition of TS after 48 h. The combination with leucovorin resulted in a more pronounced inhibition after 48 h (approximately 70%). A significant relationship was observed with outcome of treatment; when thymidylate synthase levels were high and inhibition was low, no response was observed. A separate study showed that low thymidylate synthase levels appeared to be an independent prognostic factor for adjuvant therapy. Key words: thymidylate synthase, S-fluorouracil, leucovorin, resistance, folates, antifolates EurJ Cancer, Vol. 31A, Nos 718, pp. -

Nucleotides Lymphocytes by Purine and Pyrimidine Proliferation, And
Differential Control of Cell Cycle, Proliferation, and Survival of Primary T Lymphocytes by Purine and Pyrimidine Nucleotides This information is current as of September 27, 2021. Laurence Quéméneur, Luc-Marie Gerland, Monique Flacher, Martine Ffrench, Jean-Pierre Revillard and Laurent Genestier J Immunol 2003; 170:4986-4995; ; doi: 10.4049/jimmunol.170.10.4986 Downloaded from http://www.jimmunol.org/content/170/10/4986 References This article cites 44 articles, 21 of which you can access for free at: http://www.jimmunol.org/ http://www.jimmunol.org/content/170/10/4986.full#ref-list-1 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists by guest on September 27, 2021 • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2003 by The American Association of Immunologists All rights reserved. Print ISSN: 0022-1767 Online ISSN: 1550-6606. The Journal of Immunology Differential Control of Cell Cycle, Proliferation, and Survival of Primary T Lymphocytes by Purine and Pyrimidine Nucleotides1 Laurence Que´me´neur,* Luc-Marie Gerland,† Monique Flacher,* Martine Ffrench,† Jean-Pierre Revillard,* and Laurent Genestier2* Purine and pyrimidine nucleotides play critical roles in DNA and RNA synthesis as well as in membrane lipid biosynthesis and protein glycosylation. -

Cold Spring Harbor Laboratory of Quantitative
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Cold Spring Harbor Laboratory Institutional Repository COLD SPRING HARBOR LABORATORY OF QUANTITATIVE BIOLOGY COVER PHOTO: SAND SPIT AT COLD SPRING HARBOR COLD SPRING HARBOR LABORATORY OF QUANTITATIVE BIOLOGY ESTABLISHED JULY 1, 1963 FIRST ANNUAL REPORT CALENDAR YEAR 1964 COLD SPRING HARBOR, LONG ISLAND, NEW YORK COLD SPRING HARBOR LABORATORY OF QUANTITATIVE BIOLOGY Cold Spring Harbor, New York OFFICERS OF THE CORPORATION Chairman: Dr. Edward L. Tatum 1st Vice-chairman: Dr. Harry Eagle Laboratory Director: Dr. John Cairns 2nd Vice-chairman: Mr. Walter H. Page Secretary-Treasurer: Mr. John B. Philips BOARD OF TRUSTEES AND PARTICIPATING INSTITUTIONS The Rockefeller Institute Albert Einstein College of Medicine Dr. Edward L. Tatum Dr. Harry Eagle Long Island Biological Association Mr. Walter H. Page Duke University Dr. Philip Handler Princeton University Dr. Arthur B. Pardee Brooklyn College of the City University New York University Medical Center of New York of New York University Dr. Harry G. Albaum Dr. Alan W. Bernheimer Sloan-Kettering Institute for The Public Health Research Institute Cancer Research of the City of New York, Inc. Dr. Leo Wade Dr. George K. Hirst INDIVIDUAL TRUSTEES Mr. Robert V. Lindsay Mr. Dudley W. Stoddard TERMS OF OFFICE To expire 1965 To expire 1966 To expire 1967 Dr. Harry G. Albaum Dr. George K. Hirst Dr. Alan W. Bernheimer Dr. Philip Handler Mr. Robert V. Lindsay Dr. Harry Eagle Dr. Arthur B. Pardee Dr. Edward L. Tatum -

Harold E. Varmus
RETROVIRUSES AND ONCOGENES I Nobel Lecture, December 8, 1989 by HAROLD E. VARMUS Departments of Microbiology and Immunology, and Biochemistry and Biophysics, University of California at San Francisco, San Francisco, U.S.A. INTRODUCTION The story that Mike Bishop and I will tell in these two lectures is one in which retroviruses, oncogenes, our personal histories, and the history of tumor virology are closely interwoven. It begins with some simple questions about the origin and behavior of viral genes and takes us to a vantage point from which we can survey many aspects of retroviruses and animal cells, including some of the aberrations that lead to cancer. We now know that retroviruses capture normal cellular genes and convert them to cancer- causing genes, called oncogenes. Such transductions are rare, but depend upon the normal events of an intricate virus life cycle. Retroviruses have introduced us to more than forty cellular genes with the potential to become oncogenes, some discovered as components of viral genomes, others as genetic targets for viral insertion mutations. It has been our privilege to participate in a generous share of the experiments that established these principles. But we have required the help of many talented people in our laboratories at UCSF, as well as the collaboration and friendly competition of others elsewhere. (I mention as many names as the narrative can bear, but inevitably I must apologize to valued colleagues who remain anonymous here.) Several viruses also figure in our tale, but Rous sarcoma virus again has the leading role, yet another of many tributes to the pioneering work of Peyton Rous and to the principle of delayed gratification in science.