Guidelines for Histopathological Evaluation
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Te2, Part Iii
TERMINOLOGIA EMBRYOLOGICA Second Edition International Embryological Terminology FIPAT The Federative International Programme for Anatomical Terminology A programme of the International Federation of Associations of Anatomists (IFAA) TE2, PART III Contents Caput V: Organogenesis Chapter 5: Organogenesis (continued) Systema respiratorium Respiratory system Systema urinarium Urinary system Systemata genitalia Genital systems Coeloma Coelom Glandulae endocrinae Endocrine glands Systema cardiovasculare Cardiovascular system Systema lymphoideum Lymphoid system Bibliographic Reference Citation: FIPAT. Terminologia Embryologica. 2nd ed. FIPAT.library.dal.ca. Federative International Programme for Anatomical Terminology, February 2017 Published pending approval by the General Assembly at the next Congress of IFAA (2019) Creative Commons License: The publication of Terminologia Embryologica is under a Creative Commons Attribution-NoDerivatives 4.0 International (CC BY-ND 4.0) license The individual terms in this terminology are within the public domain. Statements about terms being part of this international standard terminology should use the above bibliographic reference to cite this terminology. The unaltered PDF files of this terminology may be freely copied and distributed by users. IFAA member societies are authorized to publish translations of this terminology. Authors of other works that might be considered derivative should write to the Chair of FIPAT for permission to publish a derivative work. Caput V: ORGANOGENESIS Chapter 5: ORGANOGENESIS -

Ovarian Cancer and Cervical Cancer
What Every Woman Should Know About Gynecologic Cancer R. Kevin Reynolds, MD The George W. Morley Professor & Chief, Division of Gyn Oncology University of Michigan Ann Arbor, MI What is gynecologic cancer? Cancer is a disease where cells grow and spread without control. Gynecologic cancers begin in the female reproductive organs. The most common gynecologic cancers are endometrial cancer, ovarian cancer and cervical cancer. Less common gynecologic cancers involve vulva, Fallopian tube, uterine wall (sarcoma), vagina, and placenta (pregnancy tissue: molar pregnancy). Ovary Uterus Endometrium Cervix Vagina Vulva What causes endometrial cancer? Endometrial cancer is the most common gynecologic cancer: one out of every 40 women will develop endometrial cancer. It is caused by too much estrogen, a hormone normally present in women. The most common cause of the excess estrogen is being overweight: fat cells actually produce estrogen. Another cause of excess estrogen is medication such as tamoxifen (often prescribed for breast cancer treatment) or some forms of prescribed estrogen hormone therapy (unopposed estrogen). How is endometrial cancer detected? Almost all endometrial cancer is detected when a woman notices vaginal bleeding after her menopause or irregular bleeding before her menopause. If bleeding occurs, a woman should contact her doctor so that appropriate testing can be performed. This usually includes an endometrial biopsy, a brief, slightly crampy test, performed in the office. Fortunately, most endometrial cancers are detected before spread to other parts of the body occurs Is endometrial cancer treatable? Yes! Most women with endometrial cancer will undergo surgery including hysterectomy (removal of the uterus) in addition to removal of ovaries and lymph nodes. -

About Ovarian Cancer Overview and Types
cancer.org | 1.800.227.2345 About Ovarian Cancer Overview and Types If you have been diagnosed with ovarian cancer or are worried about it, you likely have a lot of questions. Learning some basics is a good place to start. ● What Is Ovarian Cancer? Research and Statistics See the latest estimates for new cases of ovarian cancer and deaths in the US and what research is currently being done. ● Key Statistics for Ovarian Cancer ● What's New in Ovarian Cancer Research? What Is Ovarian Cancer? Cancer starts when cells in the body begin to grow out of control. Cells in nearly any part of the body can become cancer and can spread. To learn more about how cancers start and spread, see What Is Cancer?1 Ovarian cancers were previously believed to begin only in the ovaries, but recent evidence suggests that many ovarian cancers may actually start in the cells in the far (distal) end of the fallopian tubes. 1 ____________________________________________________________________________________American Cancer Society cancer.org | 1.800.227.2345 What are the ovaries? Ovaries are reproductive glands found only in females (women). The ovaries produce eggs (ova) for reproduction. The eggs travel from the ovaries through the fallopian tubes into the uterus where the fertilized egg settles in and develops into a fetus. The ovaries are also the main source of the female hormones estrogen and progesterone. One ovary is on each side of the uterus. The ovaries are mainly made up of 3 kinds of cells. Each type of cell can develop into a different type of tumor: ● Epithelial tumors start from the cells that cover the outer surface of the ovary. -

Reproductive System, Day 2 Grades 4-6, Lesson #12
Family Life and Sexual Health, Grades 4, 5 and 6, Lesson 12 F.L.A.S.H. Reproductive System, day 2 Grades 4-6, Lesson #12 Time Needed 40-50 minutes Student Learning Objectives To be able to... 1. Distinguish reproductive system facts from myths. 2. Distinguish among definitions of: ovulation, ejaculation, intercourse, fertilization, implantation, conception, circumcision, genitals, and semen. 3. Explain the process of the menstrual cycle and sperm production/ejaculation. Agenda 1. Explain lesson’s purpose. 2. Use transparencies or your own drawing skills to explain the processes of the male and female reproductive systems and to answer “Anonymous Question Box” questions. 3. Use Reproductive System Worksheets #3 and/or #4 to reinforce new terminology. 4. Use Reproductive System Worksheet #5 as a large group exercise to reinforce understanding of the reproductive process. 5. Use Reproductive System Worksheet #6 to further reinforce Activity #2, above. This lesson was most recently edited August, 2009. Public Health - Seattle & King County • Family Planning Program • © 1986 • revised 2009 • www.kingcounty.gov/health/flash 12 - 1 Family Life and Sexual Health, Grades 4, 5 and 6, Lesson 12 F.L.A.S.H. Materials Needed Classroom Materials: OPTIONAL: Reproductive System Transparency/Worksheets #1 – 2, as 4 transparencies (if you prefer not to draw) OPTIONAL: Overhead projector Student Materials: (for each student) Reproductive System Worksheets 3-6 (Which to use depends upon your class’ skill level. Each requires slightly higher level thinking.) Public Health - Seattle & King County • Family Planning Program • © 1986 • revised 2009 • www.kingcounty.gov/health/flash 12 - 2 Family Life and Sexual Health, Grades 4, 5 and 6, Lesson 12 F.L.A.S.H. -

FEMALE REPRODUCTIVE SYSTEM Female Reproduc�Ve System
Human Anatomy Unit 3 FEMALE REPRODUCTIVE SYSTEM Female Reproducve System • Gonads = ovaries – almond shaped – flank the uterus on either side – aached to the uterus and body wall by ligaments • Gametes = oocytes – released from the ovary during ovulaon – Develop within ovarian follicles Ligaments • Broad ligament – Aaches to walls and floor of pelvic cavity – Connuous with parietal peritoneum • Round ligament – Perpendicular to broad ligament • Ovarian ligament – Lateral surface of uterus ‐ ‐> medial surface of ovary • Suspensory ligament – Lateral surface of ovary ‐ ‐> pelvic wall Ovarian Follicles • Layers of epithelial cells surrounding ova • Primordial follicle – most immature of follicles • Primary follicle – single layer of follicular (granulosa) cells • Secondary – more than one layer and growing cavies • Graafian – Fluid filled antrum – ovum supported by many layers of follicular cells – Ovum surrounded by corona radiata Ovarian Follicles Corpus Luteum • Ovulaon releases the oocyte with the corona radiata • Leaves behind the rest of the Graafian follicle • Follicle becomes corpus luteum • Connues to secrete hormones to support possible pregnancy unl placenta becomes secretory or no implantaon • Becomes corpus albicans when no longer funconal Corpus Luteum and Corpus Albicans Uterine (Fallopian) Tubes • Ciliated tubes – Passage of the ovum to the uterus and – Passage of sperm toward the ovum • Fimbriae – finger like projecons that cover the ovary and sway, drawing the ovum inside aer ovulaon The Uterus • Muscular, hollow organ – supports -

Creating an Artificial 3-Dimensional Ovarian Follicle Culture System
micromachines Article Creating an Artificial 3-Dimensional Ovarian Follicle Culture System Using a Microfluidic System Mae W. Healy 1,2, Shelley N. Dolitsky 1, Maria Villancio-Wolter 3, Meera Raghavan 3, Alexandra R. Tillman 3 , Nicole Y. Morgan 3, Alan H. DeCherney 1, Solji Park 1,*,† and Erin F. Wolff 1,4,† 1 Program in Reproductive and Adult Endocrinology, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.W.H.); [email protected] (S.N.D.); [email protected] (A.H.D.); [email protected] (E.F.W.) 2 Department of Obstetrics and Gynecology, Walter Reed National Military Medical Center, Bethesda, MD 20889, USA 3 Trans-NIH Shared Resource on Biomedical Engineering and Physical Science, National Institute of Biomedical Imaging and Bioengineering, National Institutes of Health, Bethesda, MD 20892, USA; [email protected] (M.V.-W.); [email protected] (M.R.); [email protected] (A.R.T.); [email protected] (N.Y.M.) 4 Pelex, Inc., McLean, VA 22101, USA * Correspondence: [email protected] † Solji Park and Erin F. Wolff are co-senior authors. Abstract: We hypothesized that the creation of a 3-dimensional ovarian follicle, with embedded gran- ulosa and theca cells, would better mimic the environment necessary to support early oocytes, both structurally and hormonally. Using a microfluidic system with controlled flow rates, 3-dimensional Citation: Healy, M.W.; Dolitsky, S.N.; two-layer (core and shell) capsules were created. The core consists of murine granulosa cells in Villancio-Wolter, M.; Raghavan, M.; 0.8 mg/mL collagen + 0.05% alginate, while the shell is composed of murine theca cells suspended Tillman, A.R.; Morgan, N.Y.; in 2% alginate. -
![Oogenesis [PDF]](https://docslib.b-cdn.net/cover/2902/oogenesis-pdf-452902.webp)
Oogenesis [PDF]
Oogenesis Dr Navneet Kumar Professor (Anatomy) K.G.M.U Dr NavneetKumar Professor Anatomy KGMU Lko Oogenesis • Development of ovum (oogenesis) • Maturation of follicle • Fate of ovum and follicle Dr NavneetKumar Professor Anatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Oogenesis • Site – ovary • Duration – 7th week of embryo –primordial germ cells • -3rd month of fetus –oogonium • - two million primary oocyte • -7th month of fetus primary oocyte +primary follicle • - at birth primary oocyte with prophase of • 1st meiotic division • - 40 thousand primary oocyte in adult ovary • - 500 primary oocyte attain maturity • - oogenesis completed after fertilization Dr Navneet Kumar Dr NavneetKumar Professor Professor (Anatomy) Anatomy KGMU Lko K.G.M.U Development of ovum Oogonium(44XX) -In fetal ovary Primary oocyte (44XX) arrest till puberty in prophase of 1st phase meiotic division Secondary oocyte(22X)+Polar body(22X) 1st phase meiotic division completed at ovulation &enter in 2nd phase Ovum(22X)+polarbody(22X) After fertilization Dr NavneetKumar Professor Anatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Dr Navneet Kumar Dr ProfessorNavneetKumar (Anatomy) Professor K.G.M.UAnatomy KGMU Lko Dr NavneetKumar Professor Anatomy KGMU Lko Maturation of follicle Dr NavneetKumar Professor Anatomy KGMU Lko Maturation of follicle Primordial follicle -Follicular cells Primary follicle -Zona pallucida -Granulosa cells Secondary follicle Antrum developed Ovarian /Graafian follicle - Theca interna &externa -Membrana granulosa -Antrial -

Clinical Pelvic Anatomy
SECTION ONE • Fundamentals 1 Clinical pelvic anatomy Introduction 1 Anatomical points for obstetric analgesia 3 Obstetric anatomy 1 Gynaecological anatomy 5 The pelvic organs during pregnancy 1 Anatomy of the lower urinary tract 13 the necks of the femora tends to compress the pelvis Introduction from the sides, reducing the transverse diameters of this part of the pelvis (Fig. 1.1). At an intermediate level, opposite A thorough understanding of pelvic anatomy is essential for the third segment of the sacrum, the canal retains a circular clinical practice. Not only does it facilitate an understanding cross-section. With this picture in mind, the ‘average’ of the process of labour, it also allows an appreciation of diameters of the pelvis at brim, cavity, and outlet levels can the mechanisms of sexual function and reproduction, and be readily understood (Table 1.1). establishes a background to the understanding of gynae- The distortions from a circular cross-section, however, cological pathology. Congenital abnormalities are discussed are very modest. If, in circumstances of malnutrition or in Chapter 3. metabolic bone disease, the consolidation of bone is impaired, more gross distortion of the pelvic shape is liable to occur, and labour is likely to involve mechanical difficulty. Obstetric anatomy This is termed cephalopelvic disproportion. The changing cross-sectional shape of the true pelvis at different levels The bony pelvis – transverse oval at the brim and anteroposterior oval at the outlet – usually determines a fundamental feature of The girdle of bones formed by the sacrum and the two labour, i.e. that the ovoid fetal head enters the brim with its innominate bones has several important functions (Fig. -

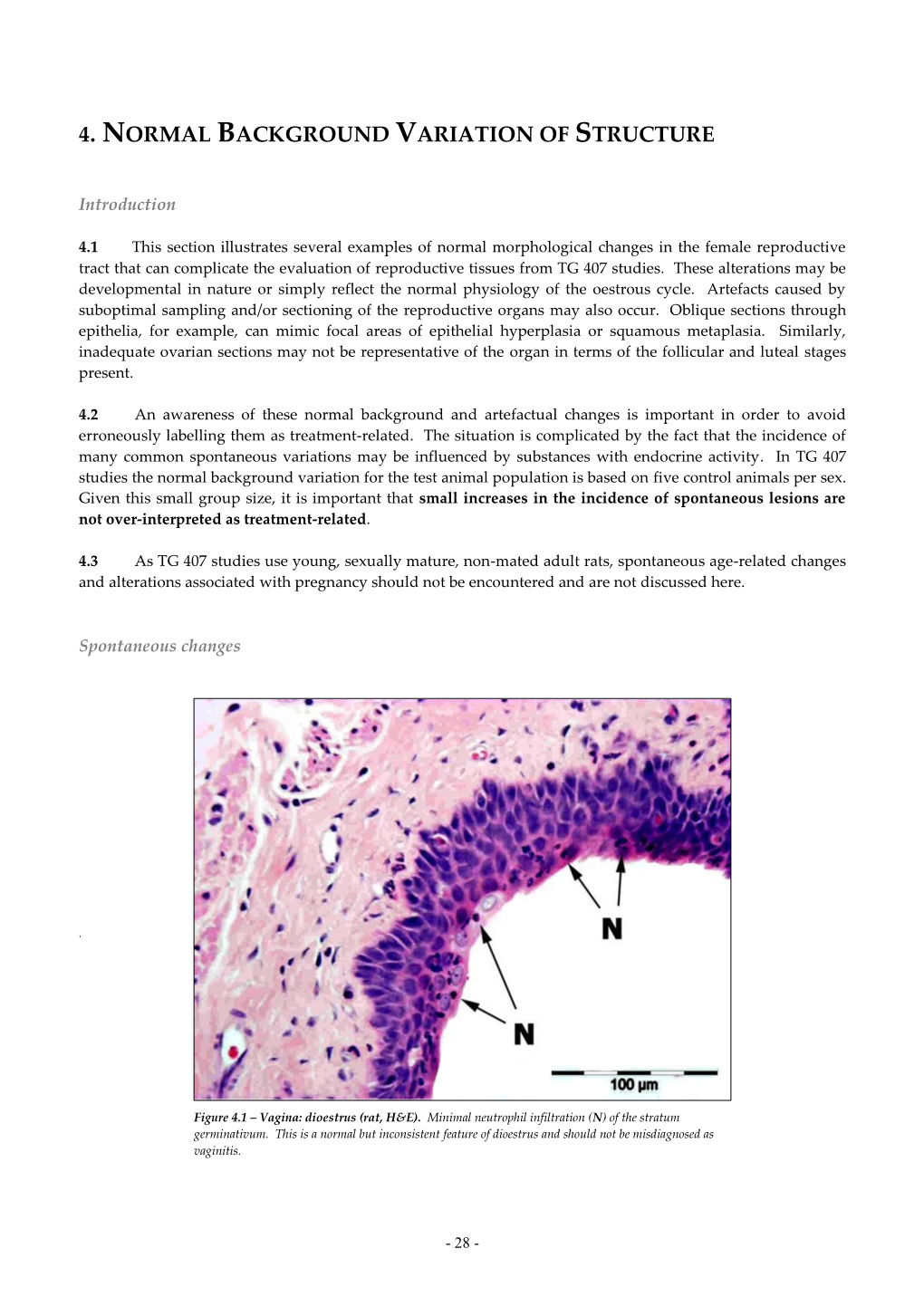
Ovary – Angiectasis
Ovary – Angiectasis Figure Legend: Figure 1 Ovary - Angiectasis, in a female F344/N rat from a chronic study. Dilated, enlarged vascular channels are present throughout the ovarian parenchyma. Figure 2 Ovary - Angiectasis in a female F344/N rat from a chronic study (higher magnification of Figure 1). Dilated, enlarged, vascular channels are present in the ovarian parenchyma, which are lined by endothelium. Comment: Angiectasis is characterized by channels of variable size and shape randomly distributed throughout the ovarian parenchyma (Figure 1 and Figure 2). These channels are lined by a single layer of flattened spindle-shaped cells and generally contain variable numbers of blood cells. This occurs in the ovaries more frequently in mice than in rats. Preexisting ovarian blood vessels become dilated and filled with blood cells, accompanied by compression of the ovarian parenchyma. This is a common age- related change; however, the mechanism has not been clearly elucidated. Angiectasis must be distinguished from ovarian angiomatous hyperplasia, hemangioma, and hemangiosarcoma. In ovarian angiectasis, the number of vessels is not increased, and the endothelial cells lining the dilated vascular channels appear normal in size, shape, and number. In angiomatous hyperplasia, there is an increase in the number of vessels, but the endothelial cells are essentially normal. In hemangioma, there is an increase in the number of vascular channels with hypertrophied endothelium. Hemangiosarcoma is characterized as a poorly delineated mass causing displacement or destruction of ovarian parenchyma, consisting of irregular, poorly formed, potentially invasive vascular channels lined by an excessive number of pleomorphic cells with generally scant cytoplasm and enlarged, oval hyperchromatic or vesicular nuclei. -

ASC-201: Avian Female Reproductive System
COOPERATIVE EXTENSION SERVICE UNIVERSITY OF KENTUCKY COLLEGE OF AGRICULTURE, FOOD AND ENVIRONMENT, LEXINGTON, KY, 40546 ASC-201 Avian Female Reproductive System Jacquie Jacob and Tony Pescatore, Animal Sciences nyone raising poultry for While mammals typically give Although the embryo has two ova- eggs, whether for eating or birth to their offpsring, the off- ries and oviducts, only the left pair forA incubation, should have an spring of birds develop outside (i.e., ovary and oviduct) develops. understanding of the reproduc- the body of the parents—in eggs. The right typically regresses during tive system. This will help them When carried in the womb, mam- development and is non-functional understand any problems that may malian embryos receive their daily in the adult bird. There have been occur and how to correct them. requirement for nutrients directly cases, however, where the left ova- The avian reproductive system is from their mother via the placenta. ry and oviduct have been damaged different from that of mammals. For birds, however, all the nutri- and the right one has developed to Nature has designed it to better ents that will be needed for the replace it. suit the risks associated with being embryo to fully develop must be Theovary is a cluster of devel- a bird. Unless you are a bird of prey provided in the egg before it is laid. oping yolks or ova and is located (a hawk, eagle or falcon), you are The female reproductive system midway between the neck and the faced with the fact that everyone is of the chicken is shown in Figure tail of the bird, attached to the trying to eat you. -

Recently Discovered Interstitial Cell Population of Telocytes: Distinguishing Facts from Fiction Regarding Their Role in The
medicina Review Recently Discovered Interstitial Cell Population of Telocytes: Distinguishing Facts from Fiction Regarding Their Role in the Pathogenesis of Diverse Diseases Called “Telocytopathies” Ivan Varga 1,*, Štefan Polák 1,Ján Kyseloviˇc 2, David Kachlík 3 , L’ubošDanišoviˇc 4 and Martin Klein 1 1 Institute of Histology and Embryology, Faculty of Medicine, Comenius University in Bratislava, 813 72 Bratislava, Slovakia; [email protected] (Š.P.); [email protected] (M.K.) 2 Fifth Department of Internal Medicine, Faculty of Medicine, Comenius University in Bratislava, 813 72 Bratislava, Slovakia; [email protected] 3 Institute of Anatomy, Second Faculty of Medicine, Charles University, 128 00 Prague, Czech Republic; [email protected] 4 Institute of Medical Biology, Genetics and Clinical Genetics, Faculty of Medicine, Comenius University in Bratislava, 813 72 Bratislava, Slovakia; [email protected] * Correspondence: [email protected]; Tel.: +421-90119-547 Received: 4 December 2018; Accepted: 11 February 2019; Published: 18 February 2019 Abstract: In recent years, the interstitial cells telocytes, formerly known as interstitial Cajal-like cells, have been described in almost all organs of the human body. Although telocytes were previously thought to be localized predominantly in the organs of the digestive system, as of 2018 they have also been described in the lymphoid tissue, skin, respiratory system, urinary system, meninges and the organs of the male and female genital tracts. Since the time of eminent German pathologist Rudolf Virchow, we have known that many pathological processes originate directly from cellular changes. Even though telocytes are not widely accepted by all scientists as an individual and morphologically and functionally distinct cell population, several articles regarding telocytes have already been published in such prestigious journals as Nature and Annals of the New York Academy of Sciences. -

Nomina Histologica Veterinaria, First Edition
NOMINA HISTOLOGICA VETERINARIA Submitted by the International Committee on Veterinary Histological Nomenclature (ICVHN) to the World Association of Veterinary Anatomists Published on the website of the World Association of Veterinary Anatomists www.wava-amav.org 2017 CONTENTS Introduction i Principles of term construction in N.H.V. iii Cytologia – Cytology 1 Textus epithelialis – Epithelial tissue 10 Textus connectivus – Connective tissue 13 Sanguis et Lympha – Blood and Lymph 17 Textus muscularis – Muscle tissue 19 Textus nervosus – Nerve tissue 20 Splanchnologia – Viscera 23 Systema digestorium – Digestive system 24 Systema respiratorium – Respiratory system 32 Systema urinarium – Urinary system 35 Organa genitalia masculina – Male genital system 38 Organa genitalia feminina – Female genital system 42 Systema endocrinum – Endocrine system 45 Systema cardiovasculare et lymphaticum [Angiologia] – Cardiovascular and lymphatic system 47 Systema nervosum – Nervous system 52 Receptores sensorii et Organa sensuum – Sensory receptors and Sense organs 58 Integumentum – Integument 64 INTRODUCTION The preparations leading to the publication of the present first edition of the Nomina Histologica Veterinaria has a long history spanning more than 50 years. Under the auspices of the World Association of Veterinary Anatomists (W.A.V.A.), the International Committee on Veterinary Anatomical Nomenclature (I.C.V.A.N.) appointed in Giessen, 1965, a Subcommittee on Histology and Embryology which started a working relation with the Subcommittee on Histology of the former International Anatomical Nomenclature Committee. In Mexico City, 1971, this Subcommittee presented a document entitled Nomina Histologica Veterinaria: A Working Draft as a basis for the continued work of the newly-appointed Subcommittee on Histological Nomenclature. This resulted in the editing of the Nomina Histologica Veterinaria: A Working Draft II (Toulouse, 1974), followed by preparations for publication of a Nomina Histologica Veterinaria.