Guide to Sampling and Identifying Larvae of Species of Maricultural
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
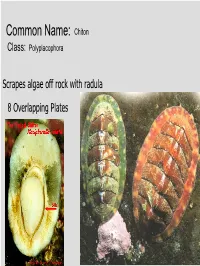
Common Name: Chiton Class: Polyplacophora
Common Name: Chiton Class: Polyplacophora Scrapes algae off rock with radula 8 Overlapping Plates Phylum? Mollusca Class? Gastropoda Common name? Brown sea hare Class? Scaphopoda Common name? Tooth shell or tusk shell Mud Tentacle Foot Class? Gastropoda Common name? Limpet Phylum? Mollusca Class? Bivalvia Class? Gastropoda Common name? Brown sea hare Phylum? Mollusca Class? Gastropoda Common name? Nudibranch Class? Cephalopoda Cuttlefish Octopus Squid Nautilus Phylum? Mollusca Class? Gastropoda Most Bivalves are Filter Feeders A B E D C • A: Mantle • B: Gill • C: Mantle • D: Foot • E: Posterior adductor muscle I.D. Green: Foot I.D. Red Gills Three Body Regions 1. Head – Foot 2. Visceral Mass 3. Mantle A B C D • A: Radula • B: Mantle • C: Mouth • D: Foot What are these? Snail Radulas Dorsal HingeA Growth line UmboB (Anterior) Ventral ByssalC threads Mussel – View of Outer Shell • A: Hinge • B: Umbo • C: Byssal threads Internal Anatomy of the Bay Mussel A B C D • A: Labial palps • B: Mantle • C: Foot • D: Byssal threads NacreousB layer Posterior adductorC PeriostracumA muscle SiphonD Mantle Byssal threads E Internal Anatomy of the Bay Mussel • A: Periostracum • B: Nacreous layer • C: Posterior adductor muscle • D: Siphon • E: Mantle Byssal gland Mantle Gill Foot Labial palp Mantle Byssal threads Gill Byssal gland Mantle Foot Incurrent siphon Byssal Labial palp threads C D B A E • A: Foot • B: Gills • C: Posterior adductor muscle • D: Excurrent siphon • E: Incurrent siphon Heart G F H E D A B C • A: Foot • B: Gills • C: Mantle • D: Excurrent siphon • E: Incurrent siphon • F: Posterior adductor muscle • G: Labial palps • H: Anterior adductor muscle Siphon or 1. -

Silicified Eocene Molluscs from the Lower Murchison District, Southern Carnarvon Basin, Western Australia
[<ecords o{ the Western A uslralian Museum 24: 217--246 (2008). Silicified Eocene molluscs from the Lower Murchison district, Southern Carnarvon Basin, Western Australia Thomas A. Darragh1 and George W. Kendrick2.3 I Department of Invertebrate Palaeontology, Museum Victoria, 1'.0. Box 666, Melbourne, Victoria 3001, Australia. Email: tdarragh(il.Illuseum.vic.gov.au :' Department of Earth and Planetary Sciences, Western Australian Museum, Locked Bag 49, Welshpool D.C., Western Australia 6986, Australia. 1 School of Earth and Ceographical Sciences, The University of Western Australia, 35 Stirling Highway, Crawlev, Western Australia 6009, Australia. Abstract - Silicified Middle to Late Eocene shallow water sandstones outcropping in the Lower Murchison District near Kalbarri township contain a silicified fossil fauna including foraminifera, sponges, bryozoans, solitary corals, brachiopods, echinoids and molluscs. The known molluscan fauna consists of 51 species, comprising 2 cephalopods, 14 bivalves, 1 scaphopod and 34 gastropods. Of these taxa three are newly described, Cerithium lvilya, Zeacolpus bartol1i, and Lyria lamellatoplicata. 25 of these molluscs are identical to or closely comparable with taxa from the southern Australian Eocene. The occurrence of this fauna extends the Southeast Australian Province during the Eocene from southwest Western Australia along the west coast north to at least 27° present day south latitude; consequently the province is here renamed the Southern Australian Province. Keywords: siliceous fossils, Eocene, Kalbarri, molluscs, new taxa, Carnarvon Basin, biogeography, Southern Australian Province. INTRODUCTION The source deposit, a pallid to ferruginous silicified Eocene marine molluscan assemblages from sandstone, forms a weakly defined, low breakaway coastal sedimentary basins in southern Australia trending N-S and sloping gently westward. -
Field Identification Guide to the Living Marine Resources In
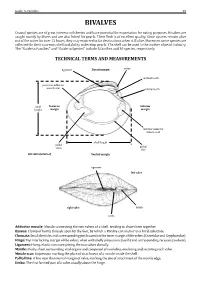
Guide to Families 29 BIVALVES Coastal species are of great interest to fisheries and have potential for exportation for eating purposes. Bivalves are caught mainly by divers and are also fished for pearls. Their flesh is of excellent quality. Since oysters remain alive out of the water for over 12 hours, they may exported to far destinations when still alive. Moreover, some species are collected for their nacreous shell and ability to develop pearls. The shell can be used in the mother of pearl industry. The “Guide to Families’’ andTECHNICAL ‘‘Guide to Species’’ TERMS include 5AND families MEASUREMENTS and 10 species, respectively. ligament Dorsal margin umbo posterior adductor cardinal tooth muscle scar lateral tooth Posterior Anterior margin margin shell height anterior adductor muscle scar pallial sinus pallial shell length line left valve (interior) Ventral margin ligament left valve right valve lunule umbo Adductor muscle: Byssus: Chomata: Muscle connecting the two valves of a shell, tending to draw them together. Hinge: Clump of horny threads spun by the foot, by which a Bivalve can anchor to a hard substrate. Ligament: Small denticles and corresponding pits located on the inner margin of the valves (Ostreidae and Gryphaeidae). Mantle: Top interlocking margin of the valves, often with shelly projections (teeth) and corresponding recesses (sockets). Muscle scar: Horny, elastic structure joining the two valves dorsally. Pallial line: Fleshy sheet surrounding vital organs and composed of two lobes, one lining and secreting each valve. Umbo: Impression marking the place of attachment of a muscle inside the shell. A line near the internal margin of valve, marking the site of attachment of the mantle edge. -

Commercial Performance of Blue Mussel (Mytilus Edulis, L.) Stocks at a Microgeographic Scale
Journal of Marine Science and Engineering Article Commercial Performance of Blue Mussel (Mytilus edulis, L.) Stocks at a Microgeographic Scale Efflam Guillou 1, Carole Cyr 2, Jean-François Laplante 2, François Bourque 3, Nicolas Toupoint 1,2,* and Réjean Tremblay 1 1 Institut des Sciences de la Mer de Rimouski (ISMER), Université du Québec à Rimouski (UQAR), 310 Allée des Ursulines, CP 3300, Rimouski, QC G5L 3A1, Canada; Effl[email protected] (E.G.); [email protected] (R.T.) 2 MERINOV, Centre d’Innovation de l’aquaculture et des pêches du Québec, Secteur Aquaculture, 107-125 Chemin du Parc, Cap-aux-Meules, QC G4T 1B3, Canada; [email protected] (C.C.); [email protected] (J.-F.L.) 3 Ministry of Agriculture, Fisheries and Food of Québec (MAPAQ - Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec), Fisheries and commercial aquaculture branch, 101-125, Chemin du Parc, Cap-aux-Meules, QC G4T 1B3, Canada; [email protected] * Correspondence: [email protected]; Tel.: +1-(418)-986-4795 (#3227) Received: 18 April 2020; Accepted: 23 May 2020; Published: 26 May 2020 Abstract: Bivalve aquaculture is an important component of the economy in eastern Canada. Because of current social, environmental, economic, and resource constraints, offshore mussel cultivation seems to be a promising strategy. With the objective of optimizing farming strategies that support the sustainability and development of the mussel industry at a microgeographic scale, we evaluated, after a traditional two year production cycle, the commercial performance of spat from several mussel (Mytilus edulis) stocks originating from sites separated by less than 65 km and cultivated at two different grow-out sites (shallow lagoon and offshore waters). -

Clam Dissection Guideline
Clam Dissection Guideline BACKGROUND: Clams are bivalves, meaning that they have shells consisting of two halves, or valves. The valves are joined at the top, and the adductor muscles on each side hold the shell closed. If the adductor muscles are relaxed, the shell is pulled open by ligaments located on each side of the umbo. The clam's foot is used to dig down into the sand, and a pair of long incurrent and excurrent siphons that extrude from the clam's mantle out the side of the shell reach up to the water above (only the exit points for the siphons are shown). Clams are filter feeders. Water and food particles are drawn in through one siphon to the gills where tiny, hair-like cilia move the water, and the food is caught in mucus on the gills. From there, the food-mucus mixture is transported along a groove to the palps (mouth flaps) which push it into the clam's mouth. The second siphon carries away the water. The gills also draw oxygen from the water flow. The mantle, a thin membrane surrounding the body of the clam, secretes the shell. The oldest part of the clam shell is the umbo, and it is from the hinge area that the clam extends as it grows. I. Purpose: The purpose of this lab is to identify the internal and external structures of a mollusk by dissecting a clam. II. Materials: 2 pairs of safety goggles 1 paper towel 2 pairs of gloves 1 pair of scissors 1 preserved clam 2 pairs of forceps 1 dissecting tray 2 probes III. -

Attachment Properties of Blue Mussel (Mytilus Edulis L.) Byssus Threads on Culture-Based Artificial Collector Substrates
Aquacultural Engineering 42 (2010) 128–139 View metadata, citation and similar papers at core.ac.uk brought to you by CORE Contents lists available at ScienceDirect provided by Electronic Publication Information Center Aquacultural Engineering journal homepage: www.elsevier.com/locate/aqua-online Attachment properties of blue mussel (Mytilus edulis L.) byssus threads on culture-based artificial collector substrates M. Brenner a,b,c,∗, B.H. Buck a,c,d a Alfred Wegener Institute for Polar and Marine Research (AWI), Am Handelshafen 12, 27570 Bremerhaven, Germany b Jacobs University Bremen, Campus Ring 1, 28759 Bremen, Germany c Institute for Marine Resourses (IMARE), Klußmannstraße 1, 27570 Bremerhaven, Germany d University of Applied Sciences Bremerhaven, An der Karlstadt 8, 27568 Bremerhaven, Germany article info abstract Article history: The attachment strength of blue mussels (Mytilus edulis) growing under exposed conditions on 10 differ- Received 25 May 2009 ent artificial substrates was measured while assessing microstructure of the applied substrate materials. Accepted 9 February 2010 Fleece-like microstructure attracted especially mussel larvae, however, most settled individuals lost attachment on this type of microstructure with increasing size during the time of experiment. Sub- Keywords: strates with thick filaments and long and fixed appendices were less attractive to larvae but provided a Spat collectors better foothold for juvenile mussels as shown by the results of the dislodgement trials. In addition these Offshore aquaculture appendices of substrates could interweave with the mussels, building up a resistant mussel/substrate con- Offshore wind farms Mytilus edulis glomerate. Our results show that a mussel byssus apparatus can withstand harsh conditions, if suitable Dislodgement substrates are deployed. -

Shellfish Regulations
Town of Nantucket Shellfishing Policy and Regulations As Adopted on March 4, 2015 by Nantucket Board of Selectmen Amended March 23, 2016; Amended April 20, 2016 Under Authority of Massachusetts General Law, Chapter 130 Under Authority of Chapter 122 of the Code of the Town of Nantucket TABLE OF CONTENTS Section 1 – Shellfishing Policy for the Town of Nantucket/Purpose of Regulations Section 2 – General Regulations (Applying to Recreational, Commercial and Aquaculture Licenses) 2.1 - License or Permit Required 2.2 - Areas Where Recreational or Commercial Shellfishing May Occur 2.3 - Daily Limit 2.4 - Landing Shellfish 2.5 - Daily Time Limit 2.6 - Closures and Red Flag 2.7 - Temperature Restrictions 2.8 - Habitat Sensitive Areas 2.9 - Bay Scallop Strandings 2.10 - Poaching 2.11 - Disturbance of Licensed or Closed Areas 2.12 - Inspection on Demand 2.13 - Possession of Seed 2.14 - Methods of Taking 2.15 – SCUBA Diving and Snorkeling 2.16 - Transplanting, Shipping, and Storing of Live Shellfish 2.16a - Transplanting Shellfish Outside Town Waters 2.16b - Shipping of Live Shellfish for Broodstock Purposes 2.16c - Transplanting Shellfish into Town Waters 2.16d - Harvesting Seed from the Wild Not Allowed 2.16e - Wet Storage of Recreational Shellfish Prohibited. 2.17 - By-Catch 2.18 - Catch Reports Provided to the Town 2.18a - Commercial Catch Reports 2.18b - Recreational Catch Reports Section 3 – Recreational (Non-commercial) Shellfishing 3.1 - Permits 3.1a - No Transfers or Refunds 3.1b - Recreational License Fees 3.2 - Cannot Harvest for Commerce -

Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora)
Gulf of Mexico Science Volume 34 Article 4 Number 1 Number 1/2 (Combined Issue) 2018 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution Martha Reguero Universidad Nacional Autónoma de México Andrea Raz-Guzmán Universidad Nacional Autónoma de México DOI: 10.18785/goms.3401.04 Follow this and additional works at: https://aquila.usm.edu/goms Recommended Citation Reguero, M. and A. Raz-Guzmán. 2018. Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution. Gulf of Mexico Science 34 (1). Retrieved from https://aquila.usm.edu/goms/vol34/iss1/4 This Article is brought to you for free and open access by The Aquila Digital Community. It has been accepted for inclusion in Gulf of Mexico Science by an authorized editor of The Aquila Digital Community. For more information, please contact [email protected]. Reguero and Raz-Guzmán: Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Lagu Gulf of Mexico Science, 2018(1), pp. 32–55 Molluscs (Mollusca: Gastropoda, Bivalvia, Polyplacophora) of Laguna Madre, Tamaulipas, Mexico: Spatial and Temporal Distribution MARTHA REGUERO AND ANDREA RAZ-GUZMA´ N Molluscs were collected in Laguna Madre from seagrass beds, macroalgae, and bare substrates with a Renfro beam net and an otter trawl. The species list includes 96 species and 48 families. Six species are dominant (Bittiolum varium, Costoanachis semiplicata, Brachidontes exustus, Crassostrea virginica, Chione cancellata, and Mulinia lateralis) and 25 are commercially important (e.g., Strombus alatus, Busycoarctum coarctatum, Triplofusus giganteus, Anadara transversa, Noetia ponderosa, Brachidontes exustus, Crassostrea virginica, Argopecten irradians, Argopecten gibbus, Chione cancellata, Mercenaria campechiensis, and Rangia flexuosa). -

Freshwater Mussels of Maritime Canada: a Flashcard Guide
Freshwater Mussels of Maritime Canada: A Flashcard Guide In Wolastoqey, Mi’kmaw, French and English UMBO DORSAL MARGIN ANTERIOR POSTERIOR MARGIN MARGIN VENTRAL MARGIN Donald F. McAlpine, Mary C. Sollows, Jacqueline B. Madill and André L. Martel ISBN 978-0-919326-80-4 All photos copyright the Canadian Museum of Nature Acknowledgements: Funding for this publication provided by the Department of Fisheries and Oceans and the New Brunswick Museum. Special thanks to Ree Brennin Houston, Department of Fisheries and Oceans; Anne Hamilton, Brent Suttie, New Brunswick Archaeological Services Branch, and indigenous language translators Allan Tremblay (Wolastoqiyk), George Paul, Howard Augustine, and Karen Narvey (Mi’kmaw). Citation: McAlpine, D.F., M.C. Sollows, J. B. Madill, and A. L. Martel. 2018. Freshwater Mussels of Maritime Canada: A Flashcard Guide in Wolastoqey, Mi’kmaw, French and English. New Brunswick Museum, Saint John, New Brunswick, and Canadian Museum of Nature, Ottawa, Canada. Use in conjunction with Martel, A. L.,D.F. McAlpine, J. Madill, D. Sabine, A. Paquet, M. Pulsifer and M. Elderkin. 2010. Pp. 551-598. Freshwater Mussels (Bivalvia: Margaritiferidae, Unionidae) of the Atlantic Maritime Ecozone. In D.F. McAlpine and I.M. Smith (eds.). Assessment of Species Diversity in the Atlantic Maritime Ecozone. NRC Research Press, National Research Council of Canada, Ottawa, ON. 785 pp. Nedeau, E.J., M.A. McCollough, and B.I. Swartz. 2000. Freshwater Mussels of Maine. Maine Department of Inland Fisheries and Wildlife, Augusta, ME, 118 pp. -

Pleistocene Molluscs from the Namaqualand Coast
ANNALS OF THE SOUTH AFRICAN MUSEUM ANNALE VAN DIE SUID-AFRIKAANSE MUSEUM Volume 52 Band July 1969 Julie Part 9 Dee! PLEISTOCENE MOLLUSCS FROM THE NAMAQUALAND COAST By A.J.CARRINGTON & B.F.KENSLEY are issued in parts at irregular intervals as material becomes available Obtainable from the South African Museum, P.O. Box 61, Cape Town word uitgegee in dele opongereelde tye na beskikbaarheid van stof OUT OF PRINT/UIT nRUK I, 2(1, 3, 5, 7-8), 3(1-2, 5, t.-p.i.), 5(2, 5, 7-9), 6(1, t.-p.i.), 7(1, 3), 8, 9(1-2), 10(1-3), 11(1-2, 7, t.-p.i.), 21, 24(2), 27, 31(1-3), 38, 44(4)· Price of this part/Prys van hierdie deel Rg.oo Trustees of the South African Museum © 1969 Printed in South Africa by In Suid-Afrika gedruk deur The Rustica Press, Pty., Ltd. Die Rustica-pers, Edms., Bpk. Court Road, Wynberg, Cape Courtweg, Wynberg, Kaap By A. ]. CARRINGTON & B. F. KENSLEY South African Museum, Cape Town (With plates 18 to 29 and I I figures) PAGE Introduction 189 Succession 190 Systematic discussion. 191 Acknowledgements 222 Summary. 222 References 223 INTRODUCTION In the course of an examination of the Tertiary to Recent sediments of the Namaqualand coast, being carried out by one of the authors (A.].C.), a collection of fossil molluscs was assembled from the Pleistocene horizons encountered in the area. The purpose of this paper is to introduce and describe some twenty species from this collection, including forms new to the South Mrican palaeontological literature. -

TREATISE ONLINE Number 48
TREATISE ONLINE Number 48 Part N, Revised, Volume 1, Chapter 31: Illustrated Glossary of the Bivalvia Joseph G. Carter, Peter J. Harries, Nikolaus Malchus, André F. Sartori, Laurie C. Anderson, Rüdiger Bieler, Arthur E. Bogan, Eugene V. Coan, John C. W. Cope, Simon M. Cragg, José R. García-March, Jørgen Hylleberg, Patricia Kelley, Karl Kleemann, Jiří Kříž, Christopher McRoberts, Paula M. Mikkelsen, John Pojeta, Jr., Peter W. Skelton, Ilya Tëmkin, Thomas Yancey, and Alexandra Zieritz 2012 Lawrence, Kansas, USA ISSN 2153-4012 (online) paleo.ku.edu/treatiseonline PART N, REVISED, VOLUME 1, CHAPTER 31: ILLUSTRATED GLOSSARY OF THE BIVALVIA JOSEPH G. CARTER,1 PETER J. HARRIES,2 NIKOLAUS MALCHUS,3 ANDRÉ F. SARTORI,4 LAURIE C. ANDERSON,5 RÜDIGER BIELER,6 ARTHUR E. BOGAN,7 EUGENE V. COAN,8 JOHN C. W. COPE,9 SIMON M. CRAgg,10 JOSÉ R. GARCÍA-MARCH,11 JØRGEN HYLLEBERG,12 PATRICIA KELLEY,13 KARL KLEEMAnn,14 JIřÍ KřÍž,15 CHRISTOPHER MCROBERTS,16 PAULA M. MIKKELSEN,17 JOHN POJETA, JR.,18 PETER W. SKELTON,19 ILYA TËMKIN,20 THOMAS YAncEY,21 and ALEXANDRA ZIERITZ22 [1University of North Carolina, Chapel Hill, USA, [email protected]; 2University of South Florida, Tampa, USA, [email protected], [email protected]; 3Institut Català de Paleontologia (ICP), Catalunya, Spain, [email protected], [email protected]; 4Field Museum of Natural History, Chicago, USA, [email protected]; 5South Dakota School of Mines and Technology, Rapid City, [email protected]; 6Field Museum of Natural History, Chicago, USA, [email protected]; 7North -

Maine Shellfish Research Compendium
March 1, 2018 SHELLFISH RESEARCH COMPENDIUM A snapshot of recent and ongoing research projects in Maine and New England’s bivalve fisheries Executive Summary: Maine Bivalve Shellfish Research Compendium (March 2018) Maine’s bivalve fisheries, including wild and cultured populations of soft-shell clams, hard clams, blue mussels, American or Eastern Oyster, European oysters and Atlantic razor clams are a valuable and essential part of coastal ecosystems, economies, and cultures. For example, the soft-shell clam fishery is consistently the second or third largest fishery in the state by total landings value and number of licenses. Further, markets for oysters and mussels grown in aquaculture settings continue to expand. The multiple values of bivalves are increasingly threatened by a host of environmental and social changes, such as warming ocean temperatures, increased predation by species such as the invasive European green crab, water pollution, changes in ocean chemistry, harmful algae blooms, changing demographics and coastal access, and challenges related to human health and well-being. The need for applied and basic research on the ecosystems that sustain these fisheries, as well research to understand and strengthen the economies and communities that rely on them has never been greater. Fortunately, as the following table illustrates, there are researchers who are conducting diverse studies to understand and improve bivalve fisheries. The following studies are all focused specifically on Maine and New England bivalve species and shellfish communities. Many of these projects have been conducted in partnership with diverse groups who intend to link the research with management and business innovations. Together, the collection of studies helps us understand important trends in bivalve fisheries and how: • clams have developed an efficient way to warn each other about predators, helping them save energy they need to grow.