Species; Each from Different Spectrums of the Animal Kingdom
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Kenya Soe Ch4 A
PART 2 STATE OF THE ENVIRONMENT 61 CHAPTER BIODIVERSITY4 Introduction The Convention on Biological Diversity (CBD) defi nes biodiversity as Kenya’s rich biodiversity Lead Authors ‘the variability among living organisms from all sources including, can be attributed to a number Ali A. Ali and Monday S. Businge among others, terrestrial, marine and other aquatic ecosystems and of factors, including a long Contributing Authors S. M. Mutune, Jane Kibwage, Ivy Achieng, the ecological complexes of which they are part [and] includes diversity evolutionary history, variable Godfrey Mwangi, David Ongare, Fred Baraza, within species, between species and of ecosystems.’ Biodiversity climatic conditions, and diverse Teresa Muthui, Lawrence M. Ndiga, Nick Mugi therefore comprises genetic and species diversity of animals and plants habitat types and ecosystems. Reviewer as well as ecosystem diversity. Kenya is endowed with an enormous The major biodiversity Nathan Gichuki diversity of ecosystems and wildlife species which live in the terrestrial, concentration sites fall within aquatic and aerial environment. These biological resources are the existing protected areas fundamental to national prosperity as a source of food, medicines, network (national parks, reserves and sanctuaries) which are mostly energy, shelter, employment and foreign exchange. For instance, managed by the Kenya Wildlife Service (KWS). However, over 70 percent agricultural productivity and development are dependent on the of the national biodiversity occurs outside the protected areas. availability of a wide variety of plant and animal genetic resources and In spite of its immense biotic capital, Kenya experiences severe on the existence of functional ecological systems, especially those that ecological and socio-economic problems. -

Field Identification Guide to the Sharks and Rays of the Red Sea and Gulf of Aden
FAO SPECIES IDENTIFICATION GUIDE FOR FISHERY PURPOSES ISSN 1020-6868 FIELD IDENTIFICATION GUIDE TO THE SHARKS AND RAYS OF THE RED SEA AND GULF OF ADEN PERSGA FAO SPECIES IDENTIFICATION GUIDE FOR FISHERY PURPOSES FIELD IDENTIFICATION GUIDE TO THE SHARKS AND RAYS OF THE RED SEA AND GULF OF ADEN by Ramón Bonfil Marine Program Wildlife Conservation Society Bronx, New York, USA and Mohamed Abdallah Strategic Action Program Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden Jeddah, Saudi Arabia FOOD AND AGRICULTURE ORGANIZATION OF THE UNITED NATIONS Rome, 2004 iii PREPARATION OF THIS DOCUMENT This document was prepared under the coordination of the Species Identification and Data Programme of the Marine Resources Service, Fishery Resources and Environment Division, Fisheries Department, Food and Agriculture Organization of the United Nations (FAO). This field guide is largely based on material prepared for training courses on elasmobranch identification delivered in the region by the first author, and promoted by the Regional Organization for the Conservation of the Environment of the Red Sea and Gulf of Aden (PERSGA), as an activity of PERSGA’s Strategic Action Programme (SAP) towards capacity building and technical assistance in the Red Sea and Gulf of Aden region. Printing was supported by Japanese Government funds. The increasing recognition of the significance of sharks and batoid fishes as ecosystem health indicators, as well as their particular importance in exploited ecosystems in the Red Sea and the Gulf of Aden, have been key considerations to promote the preparation of this Field Guide. Furthermore, in recent years the reported catches of elasmobranchs in the Red Sea and the Gulf of Aden showed a marked increase. -

Housing, Husbandry and Welfare of a “Classic” Fish Model, the Paradise Fish (Macropodus Opercularis)
animals Article Housing, Husbandry and Welfare of a “Classic” Fish Model, the Paradise Fish (Macropodus opercularis) Anita Rácz 1,* ,Gábor Adorján 2, Erika Fodor 1, Boglárka Sellyei 3, Mohammed Tolba 4, Ádám Miklósi 5 and Máté Varga 1,* 1 Department of Genetics, ELTE Eötvös Loránd University, Pázmány Péter stny. 1C, 1117 Budapest, Hungary; [email protected] 2 Budapest Zoo, Állatkerti krt. 6-12, H-1146 Budapest, Hungary; [email protected] 3 Fish Pathology and Parasitology Team, Institute for Veterinary Medical Research, Centre for Agricultural Research, Hungária krt. 21, 1143 Budapest, Hungary; [email protected] 4 Department of Zoology, Faculty of Science, Helwan University, Helwan 11795, Egypt; [email protected] 5 Department of Ethology, ELTE Eötvös Loránd University, Pázmány Péter stny. 1C, 1117 Budapest, Hungary; [email protected] * Correspondence: [email protected] (A.R.); [email protected] (M.V.) Simple Summary: Paradise fish (Macropodus opercularis) has been a favored subject of behavioral research during the last decades of the 20th century. Lately, however, with a massively expanding genetic toolkit and a well annotated, fully sequenced genome, zebrafish (Danio rerio) became a central model of recent behavioral research. But, as the zebrafish behavioral repertoire is less complex than that of the paradise fish, the focus on zebrafish is a compromise. With the advent of novel methodologies, we think it is time to bring back paradise fish and develop it into a modern model of Citation: Rácz, A.; Adorján, G.; behavioral and evolutionary developmental biology (evo-devo) studies. The first step is to define the Fodor, E.; Sellyei, B.; Tolba, M.; housing and husbandry conditions that can make a paradise fish a relevant and trustworthy model. -

Spatial Predictive Distribution Modelling of Madeira's Endemic
DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dinarte Nuno Freitas Teixeira 2009 REGIÃO AUTÓNOMA DA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA MADEIRA FSE DEPARTAMENTO DE ZOOLOGIA FACULDADE DE CIÊNCIAS E TECNOLOGIA UNIVERSIDADE DE COIMBRA Spatial predictive distribution modelling of Madeira’s endemic land snail species Dissertação apresentada à Universidade de Coimbra para cumprimento dos requisitos necessários à obtenção do grau de Mestre em Ecologia, realizada sob a orientação científica do Professor Doutor José Paulo Sousa (Universidade de Coimbra) e do Professor Doutor José Manuel Jesus (Universidade da Madeira). Dinarte Nuno Freitas Teixeir a 2009 O presente trabalho foi financiado pelo Centro de Ciência e Tecnologia da Madeira (CITMA), através da bolsa de Mestrado FSE BM I/2008 – 531, ao abrigo do Programa Operacional de Valorização do Potencial Humano e Coesão Social da RAM (RUMOS). REGIÃO AUTÓNOMA DA MADEIRA REPÚBLICA PORTUGUESA UNIÃO EUROPEIA FSE À Susana AGRADECIMENTOS Esta tese é o resultado de um trabalho conjunto para o qual muitos contribuíram e aos quais desejo reconhecer e deixar o meu agradecimento. Ao professor Doutor José Paulo Sousa, meu orientador, pela indispensável ajuda, paciência e orientação científica. Ao professor Doutor José Manuel Jesus, meu orientador, pela amizade e apoio desde os primeiros momentos. Pelo seu empenho, conselhos transmitidos, chamadas à razão e orientação científica o meu muito obrigado. Ao Doutor Pedro Cardoso, meu orientador e a quem muito devo, pelo constante acompanhamento e disponibilidade, amizade e orientação científica. Por tudo o que me ensinou, pela motivação e animo que sempre me transmitiu, e, acima de tudo, pela manutenção da objectividade do trabalho. -

Amaia Caro Aramendia
The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika PhD thesis Vitoria-Gasteiz, 2019 Amaia Caro Aramendia The genus Pyrenaearia (Gastropoda, Helicoidea): Molecular and Morphological Systematics, Biogeography and Population Dynamics Pyrenaearia generoa (Gastropoda, Helicoidea): Sistematika Molekularra eta Morfologikoa, Biogeografia eta Populazio Dinamika A thesis submitted by Amaia Caro Aramendia for the degree of Doctor of Philosophy, under the supervision of Dr. Benjamín Juan Gómez-Moliner and Dr. María José Madeira University of the Basque Country, Vitoria-Gasteiz, 2019 Zoologia eta Animalia Biologia Zelulen Saila Dpto. Zoología y Biología Celular Animal (cc)2019 AMAIA CARO ARAMENDIA (cc by-nc-nd 4.0) Astiro igo, barraskilotxo Fuji mendia da hau! Kobayashi Issa-ren haikua To the little things that run the world Esker onak Acknowledgements Tesi bat ez da pertsona bakar batena, bidean zehar laguntzen duten pertsona guztiei esker sortutako lana da eta, beraz, lehen orriek haien laguntza eskertzeko izan behar dute: En primer lugar me gustaría agradecer a mis directores, Benjamín Gómez-Moliner y María José Madeira. A Benjamín, por darme la oportunidad de entrar en el grupo de investigación y confiar en que podría realizar esta tesis. Gracias por compartir tus extensos conocimientos y por descubrirme el mundo de la malacología, que sin duda no habría encontrado por mi cuenta y ha resultado de lo más interesante. A Marijo, porque desde el principio y hasta el final has estado siempre ahí para guiarme, animarme y para ayudarme en todo lo que hiciese falta pero, sobre todo, por mostrarme que es posible compaginar este trabajo con una vida fuera de él. -

Analysis of the Diet Between an Invasive and Native Fishes in the Peruvian Amazon. Anthony Mazeroll [email protected] Introduct
Analysis of the diet between an invasive and native fishes in the Peruvian Amazon. Anthony Mazeroll [email protected] Introduction Non-native species are the second greatest threat to global species biodiversity after land development (Vitousek, D'Antonio, Loope, Rejmanek, & Westbrooks, 1997). Due to the magnitude of this threat, it is vital that the impacts of exotic species are understood. Fish biodiversity is especially threatened by the ecological changes caused by non-native species (Gozlan, Britton, Cowx, & Copp, 2010). Having higher species diversity allows more ecological processes to take place, protecting the resources of that system, and making it more resilient to change (Folke, 2006). Adding outside inputs, such as additional non-native species, into an ecosystem can have beneficial or compromising effects depending on the amount and type. Invasive species have been shown to reduce native species richness and diversity of native organisms. Some have argued that the transportation of species from one ecosystem to another is actually beneficial for diversity, and non-native species are not really an environmental “problem”. Introductions of non-native species increase diversity at a local level because in the short term there will be a lag time where both non-native and natives can coexist (Lodge, Stein, Brown, Covich, Bronmark, Garvey and Klosiewski, 1998). Species entering a new ecosystem have to pass through a gauntlet of barriers before they can become established. The usual progression of invasion is: transportation to the new ecosystem, initial establishment, spread to a larger region, and then naturalization into the new community (Marchetti, Light, Moyle, and Viers, 2004). -
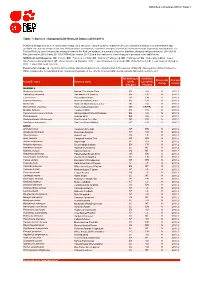
Table 7: Species Changing IUCN Red List Status (2010-2011)
IUCN Red List version 2011.2: Table 7 Table 7: Species changing IUCN Red List Status (2010-2011) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2010 (IUCN Red List version 2010.4) and 2011 (IUCN Red List version 2011.2) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.) IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2010) List (2011) change version Category Category MAMMALS Bradypus torquatus Maned Three-toed Sloth EN VU N 2011.1 Callicebus oenanthe San Martin Titi Monkey EN CR N 2011.1 Equus ferus Przewalski's Horse CR EN G 2011.2 -

Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997
The IUCN Species Survival Commission Elasmobranch Biodiversity, Conservation and Management Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 Edited by Sarah L. Fowler, Tim M. Reed and Frances A. Dipper Occasional Paper of the IUCN Species Survival Commission No. 25 IUCN The World Conservation Union Donors to the SSC Conservation Communications Programme and Elasmobranch Biodiversity, Conservation and Management: Proceedings of the International Seminar and Workshop, Sabah, Malaysia, July 1997 The IUCN/Species Survival Commission is committed to communicate important species conservation information to natural resource managers, decision-makers and others whose actions affect the conservation of biodiversity. The SSC's Action Plans, Occasional Papers, newsletter Species and other publications are supported by a wide variety of generous donors including: The Sultanate of Oman established the Peter Scott IUCN/SSC Action Plan Fund in 1990. The Fund supports Action Plan development and implementation. To date, more than 80 grants have been made from the Fund to SSC Specialist Groups. The SSC is grateful to the Sultanate of Oman for its confidence in and support for species conservation worldwide. The Council of Agriculture (COA), Taiwan has awarded major grants to the SSC's Wildlife Trade Programme and Conservation Communications Programme. This support has enabled SSC to continue its valuable technical advisory service to the Parties to CITES as well as to the larger global conservation community. Among other responsibilities, the COA is in charge of matters concerning the designation and management of nature reserves, conservation of wildlife and their habitats, conservation of natural landscapes, coordination of law enforcement efforts as well as promotion of conservation education, research and international cooperation. -

Fundamentals of Fisheries Biology
FT 273 - FUNDAMENTALS OF FISHERIES BIOLOGY 9 March 2015 Reproduction TOPICS WE WILL COVER REGARDING REPRODUCTION Reproductive anatomy Breeding behavior Development Physiological adaptations Bioenergetics Mating systems Alternative reproductive strategies Sex change REPRODUCTION OVERVIEW Reproduction is a defining feature of a species and it is evident in anatomical, behavioral, physiological and energetic adaptations Success of a species depends on ability of fish to be able to reproduce in an ever changing environment REPRODUCTION TERMS Fecundity – Number of eggs in the ovaries of the female. This is most common measure to reproductive potential. Dimorphism – differences in size or body shape between males and females Dichromatism – differences in color between males and females Bioenergetics – the balance of energy between growth, reproduction and metabolism REPRODUCTIVE ANATOMY Different between sexes Different depending on the age/ size of the fish May only be able to determine by internal examination Reproductive tissues are commonly paired structures closely assoc with kidneys FEMALE OVARIES (30 TO 70%) MALE TESTES (12% OR <) Anatomy hagfish, lamprey: single gonads no ducts; release gametes into body cavity sharks: paired gonads internal fertilization sperm emitted through cloaca, along grooves in claspers chimaeras, bony fishes: paired gonads external and internal fertilization sperm released through separate opening most teleosts: ova maintained in continuous sac from ovary to oviduct exceptions: Salmonidae, Anguillidae, Galaxidae, -

The Effects of Fires on Plants and Wildlife Species Diversity and Soil Physical and Chemical Properties at Aberdare Ranges, Kenya
THE EFFECTS OF FIRES ON PLANTS AND WILDLIFE SPECIES DIVERSITY AND SOIL PHYSICAL AND CHEMICAL PROPERTIES AT ABERDARE RANGES, KENYA WANGARI FAITH NJERI BSC HONS (2011) A THESIS SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE IN BIOLOGY OF CONSERVATION SCHOOL OF BIOLOGICAL SCIENCES, THE UNIVERSITY OF NAIROBI. 2016 DECLARATION This thesis is my original work and has not been presented for a degree in any other University Wangari Faith Njeri Signature........................................................ Date................................................................. This thesis has been submitted for examination with our approval as the University Supervisors Dr. J. M. Githaiga School of Biological Sciences University of Nairobi Signature........................................................ Date................................................................. Mr. Aggrey K. Mwala Department Of Land Resource Management & Agricultural Technology University Of Nairobi Signature........................................................ Date................................................................. ii ACKNOWLEDGEMENT I would like to express my sincere gratitude to Dr. Githaiga and Mr. Mwala, my supervisors, for the great support and guidance they accorded me throughout this research project, without which this research project would not have been successful. I would also like to thank Kenya Forest Service staff for allowing me to carry out my research work at the Aberdares forest, offering me accommodation at the station and for providing security personnel to guard me during my visits to the forest. I would also like to thank Masinde Muliro University of Science and Technology, for enabling me pursue my Masters degree by awarding me a full scholarship for the two years of study. I am very grateful to my dear mother and my entire family for the great support they offered me, both financially and morally. -

Ultimate Kenya
A pair of fantastic Sokoke Scops Owls. (DLV). All photos taken by DLV during the tour. ULTIMATE KENYA 1 – 20 / 25 APRIL 2017 LEADER: DANI LOPEZ-VELASCO Kenya lived up to its reputation of being one of the most diverse birding destinations on our planet. Once again, our Ultimate Kenya recorded a mind-boggling total of more than 750 species. This was despite the fact that we were prioritizing Kenyan specialities (a task in which we were extremely successful) rather than going all out for a huge list! 1 BirdQuest Tour Report: Ultimate Kenya www.birdquest-tours.com The first leg of our epic adventure saw us focusing on the Arabuko-Sokoke Forest where the birding is tough but the rewards are great. Over the course of the two and a half days our talented local guide helped us find all of the main specialities, with the exception of the difficult Clarke’s Weavers, which were presumably on their recently discovered breeding grounds in marshes to the north. Crested Guineafowl and Northern Carmine Bee-eater. We spent much time creeping along sandy tracks, gradually finding our targets one by one. We succeeded in getting great views of a number of skulkers, including a rather showy East Coast Akalat on our last afternoon, some reclusive Eastern Bearded Scrub Robins, a very obliging Red-tailed Ant Thrush and skulking Fischer’s and Tiny Greenbuls. Once in the Brachystegia we kept our eyes and ears open for roving flocks of flock-leader Retz’s and Chestnut-fronted Helmet Shrikes, and with these we found awkward Mombasa Woodpeckers and a single Green-backed Woodpecker, and a variety of smaller species including Black-headed Apalis, Green Barbet, Eastern Green Tinkerbird, dainty Little Yellow Flycatchers, Forest Batis, Pale Batis, cracking little Amani and Plain-backed Sunbirds and Dark-backed Weaver. -

Evolution, Culture, and Care for Betta Splendens1 Craig Watson, Matthew Dimaggio, Jeffrey Hill, Quenton Tuckett, and Roy Yanong2
FA212 Evolution, Culture, and Care for Betta splendens1 Craig Watson, Matthew DiMaggio, Jeffrey Hill, Quenton Tuckett, and Roy Yanong2 The commercial betta, or Siamese fighting fish (Betta to have shaped the evolution of labyrinth fishes, a group splendens), is one of a group of fishes called the anabantoids that formed ~60 million years ago. Life in hypoxic environ- (suborder Anabantoidei), most of which occur in fresh ments appears to have been the driving force behind the waters of Africa and southern Asia. There are roughly 137 evolutionary diversification of labyrinth fishes, including labyrinth fishes in three families, Anabantidae (28 species), the genus Betta, the most diverse group within the family Helostomatidae (1 species), and Osphronemidae (108 Osphronemidae with over 73 species. A variety of behav- species including B. splendens). The anabantoids are also ioral, morphological, and physiological traits evolved in known as labyrinth fishes, which, unlike most other fishes, response to development of air breathing as an adaptation often do not rely primarily on the gills for respiration. The to living in a hypoxic environment. While the labyrinth gills of labyrinth fishes are relatively small and primarily organ may be one of the most obvious traits, others, such as excrete the waste products ammonia and carbon dioxide. bubble nest building for reproduction, are also associated In fact, many labyrinth fishes are obligate air breathers, with this adaptation. The bubble nest allows eggs to develop meaning they must breathe at the surface to survive. in environments with elevated temperature and low pH and Other fishes have evolved a number of solutions to allow dissolved oxygen, relatively free of predators.