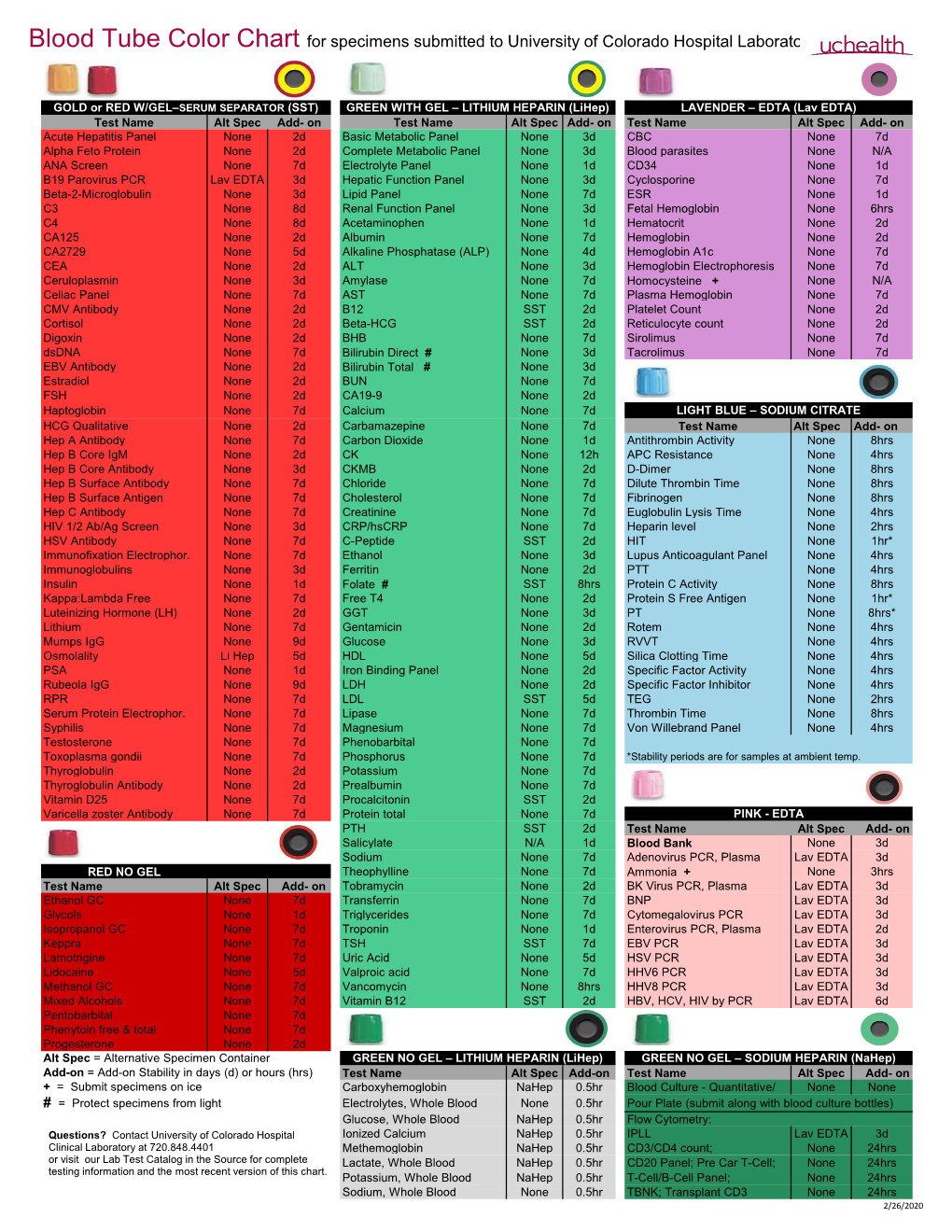
Blood Tube Color Chart for Specimens Submitted to University of Colorado Hospital Laboratory
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Ptvg-HP Vaccine Protocol
Version 10/7/2015 Randomized Phase II Trial of a DNA Vaccine Encoding Prostatic Acid Phosphatase (pTVG-HP) versus GM-CSF Adjuvant in Patients with Non-Metastatic Prostate Cancer CO 08801 Investigational Agent: BB IND 12109 - pTVG-HP DNA encoding human prostatic acid phosphatase Study Sponsors: 56 patients – treated at UWCCC and UCSF Department of Defense Prostate Cancer Research Program federal grant 50 patients in biomarker cohort – treated at UWCCC, UCSF and JHU Madison Vaccines Inc. (MVI) corporate sponsor STUDY SITE INFORMATION Study Sites: University of Wisconsin Carbone Cancer Center (UWCCC) 1111 Highland Avenue Madison, WI 53705 University of California San Francisco (UCSF) Johns Hopkins School of Medicine (JHU) Study Principal Investigator: Douglas G. McNeel, M.D., Ph.D. UWCCC 7007 Wisconsin Institutes for Medical Research 1111 Highland Ave. Madison, WI 53705 Tel: (608) 263-4198 Fax: (608) 265-0614 [email protected] UWCCC Local Principal Investigator : Glenn Liu, MD UWCCC 7051 Wisconsin Institutes for Medical Research 1111 Highland Ave. Madison, WI 53705 Tel : (608) 265-8689 Fax : (608) 265-5146 [email protected] Medical Monitor: Mark Albertini, M.D. UWCCC 600 Highland Ave. K6/5 CSC Madison, WI 53792 Tel: (608) 265-8131 Fax: (608) 265-8133 [email protected] Other UWCCC Investigators: Joshua Lang, M.D. – Clinical Investigator Christos Kyriakopolous, M.D. – Clinical Investigator Robert Jeraj, Ph.D. – Medical Physics, Quantitative Total Bone Imaging Scott Perlman, M.D. – Nuclear Medicine UCSF Principal Investigator: Lawrence Fong, M.D. JHU Principal Investigator: Emmanuel Antonarakis, MD Study Coordinator: Mary Jane Staab, R.N. B.S.N. UWCCC 600 Highland Ave. -

224 Subpart H—Hematology Kits and Packages
§ 864.7040 21 CFR Ch. I (4–1–02 Edition) Subpart H—Hematology Kits and the treatment of venous thrombosis or Packages pulmonary embolism by measuring the coagulation time of whole blood. § 864.7040 Adenosine triphosphate re- (b) Classification. Class II (perform- lease assay. ance standards). (a) Identification. An adenosine [45 FR 60611, Sept. 12, 1980] triphosphate release assay is a device that measures the release of adenosine § 864.7250 Erythropoietin assay. triphosphate (ATP) from platelets fol- (a) Identification. A erythropoietin lowing aggregation. This measurement assay is a device that measures the is made on platelet-rich plasma using a concentration of erythropoietin (an en- photometer and a luminescent firefly zyme that regulates the production of extract. Simultaneous measurements red blood cells) in serum or urine. This of platelet aggregation and ATP re- assay provides diagnostic information lease are used to evaluate platelet for the evaluation of erythrocytosis function disorders. (increased total red cell mass) and ane- (b) Classification. Class I (general mia. controls). (b) Classification. Class II. The special [45 FR 60609, Sept. 12, 1980] control for this device is FDA’s ‘‘Docu- ment for Special Controls for Erythro- § 864.7060 Antithrombin III assay. poietin Assay Premarket Notification (a) Identification. An antithrombin III (510(k)s).’’ assay is a device that is used to deter- [45 FR 60612, Sept. 12, 1980, as amended at 52 mine the plasma level of antithrombin FR 17733, May 11, 1987; 65 FR 17144, Mar. 31, III (a substance which acts with the 2000] anticoagulant heparin to prevent co- agulation). This determination is used § 864.7275 Euglobulin lysis time tests. -

Tumor Markers
Tumor Markers Alan H.B. Wu, Ph.D. Professor, Laboratory Medicine, UCSF Section Chief, Clinical Chemistry, Toxicology, Pharmacogenomics Laboratory, SFGH Learning objectives • Know the ideal characteristics of a tumor marker • Understand the role of tumor markers for diagnosis and management of patients with cancer. • Know the emerging technologies for tumor markers • Understand the role of tumor markers for therapeutic selection How do we diagnose cancer today? Physical Examination Blood tests CT scans Biopsy Human Prostate Cancer Normal Blood Smear Chronic Myeloid Leukemia Death rates for cancer vs. heart disease New cancer cases per year Cancer Site or Type New Cases Prostate 218,000 Lung 222,500 Breast 207,500 Colorectal 149,000 Urinary system 131,500 Skin 68,770 Pancreas 43,100 Ovarian 22,000 Myeloma 20,200 Thyroid 44,700 Germ Cell 9,000 Types of Tumor Markers • Hormones (hCG; calcitonin; gastrin; prolactin;) • Enzymes (acid phosphatase; alkaline phosphatase; PSA) • Cancer antigen proteins & glycoproteins (CA125; CA 15.3; CA19.9) • Metabolites (norepinephrine, epinephrine) • Normal proteins (thyroglobulin) • Oncofetal antigens (CEA, AFP) • Receptors (ER, PR, EGFR) • Genetic changes (mutations/translocations, etc.) Characteristics of an ideal tumor marker • Specificity for a single type of cancer • High sensitivity and specificity for cancerous growth • Correlation of marker level with tumor size • Homogeneous (i.e., minimal post-translational modifications) • Short half-life in circulation Roles for tumor markers • Determine risk (PSA) -

LEUKOCYTE SURFACE ORIGIN of HUMAN At-ACID GLYCOPROTEIN (OROSOMUCOID)*
LEUKOCYTE SURFACE ORIGIN OF HUMAN at-ACID GLYCOPROTEIN (OROSOMUCOID)* BY CARL G. GAHMBERG AND LEIF C. ANDERSSON (From the Department of Bacteriology and Immunology, and the Transplantation Laboratory, Department of Surgery IV, University of Helsinki, Helsinki 29, Finland) Human al-acid glycoprotein (orosomucoid) (o~I-AG)1 constitutes the main component of the seromucoid fraction of human plasma. It belongs to the acute phase proteins, which increase under conditions such as inflammation, pregnancy, and cancer (1, 2). al-AG has previously been found to be synthesized in liver (3), and after removal of terminal sialic acids, it is cleared from the circulation by binding to a receptor protein on liver cell plasma membranes (4). The structure of al-AG is well known. It is composed of a single polypeptide chain and contains 245% carbohydrate including a large amount of sialic acid. The carbohydrate is located in the first half of the peptide chain linked to asparagine residues (5, 6). The function of al-AG is unclear. However, Schmid et al. (5) and Ikenaka et al. (7) and reported that the amino acid sequence of the protein shows a significant homology with human IgG. This finding and the striking increase in inflammatory and lymphopro- liferative disorders made us consider the possibility that leukocytes could be directly involved in the synthesis and release of a~-AG. We report here the presence of a membrane form of al-AG, with an apparent tool wt of 52,000, on normal human lymphocytes, granulocytes, and monocytes. By the use of internal labeling with [3H]leucine in vitro, we demonstrate that the membrane protein is synthesized by lymphocytes. -

Endocrinology Test List Endocrinology Test List
For Endocrinologists Endocrinology Test List Endocrinology Test List Extensive Capabilities Managing patients with endocrine disorders is complex. Having access to the right test for the right patient is key. With a legacy of expertise in endocrine laboratory diagnostics, Quest Diagnostics offers an extensive menu of laboratory tests across the spectrum of endocrine disorders. This test list highlights the extensive menu of laboratory diagnostic tests we offer, including highly specialized tests and those performed using highly specific and sensitive mass spectrometry detection. It is conveniently organized by glandular function or common endocrine disorder, making it easy for you to identify the tests you need to care for the patients you treat. Comprehensive Care Quest Diagnostics Nichols Institute has been pioneering state-of-the-art endocrine testing for over four decades. Our commitment to innovative diagnostics and our dedication to quality and service means we deliver solutions that enable you to make informed clinical decisions for comprehensive patient management. We strive to remain at the forefront of innovation in endocrine testing so you can deliver the highest level of patient care. Abbreviations and Footnotes NDM, neonatal diabetes mellitus; MODY, maturity-onset diabetes of the young; CH, congenital hyperinsulinism; MSUD, maple syrup urine disease; IHH, idiopathic hypogonadotropic hypogonadism; BBS, Bardet-Biedl syndrome; OI, osteogenesis imperfecta; PKD, polycystic kidney disease; OPPG, osteoporosis-pseudoglioma syndrome; CPHD, combined pituitary hormone deficiency; GHD, growth hormone deficiency. The tests highlighted in green are performed using highly specific and sensitive mass spectrometry detection. Panels that include a test(s) performed using mass spectrometry are highlighted in yellow. For tests highlighted in blue, refer to the Athena Diagnostics website (athenadiagnostics.com/content/test-catalog) for test information. -

Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes
Tohoku J. Exp. Med., 2011,Differential 223, 161-176 Gene Expression in OPCs, Oligodendrocytes and Type II Astrocytes 161 Differential Gene Expression in Oligodendrocyte Progenitor Cells, Oligodendrocytes and Type II Astrocytes Jian-Guo Hu,1,2,* Yan-Xia Wang,3,* Jian-Sheng Zhou,2 Chang-Jie Chen,4 Feng-Chao Wang,1 Xing-Wu Li1 and He-Zuo Lü1,2 1Department of Clinical Laboratory Science, The First Affiliated Hospital of Bengbu Medical College, Bengbu, P.R. China 2Anhui Key Laboratory of Tissue Transplantation, Bengbu Medical College, Bengbu, P.R. China 3Department of Neurobiology, Shanghai Jiaotong University School of Medicine, Shanghai, P.R. China 4Department of Laboratory Medicine, Bengbu Medical College, Bengbu, P.R. China Oligodendrocyte precursor cells (OPCs) are bipotential progenitor cells that can differentiate into myelin-forming oligodendrocytes or functionally undetermined type II astrocytes. Transplantation of OPCs is an attractive therapy for demyelinating diseases. However, due to their bipotential differentiation potential, the majority of OPCs differentiate into astrocytes at transplanted sites. It is therefore important to understand the molecular mechanisms that regulate the transition from OPCs to oligodendrocytes or astrocytes. In this study, we isolated OPCs from the spinal cords of rat embryos (16 days old) and induced them to differentiate into oligodendrocytes or type II astrocytes in the absence or presence of 10% fetal bovine serum, respectively. RNAs were extracted from each cell population and hybridized to GeneChip with 28,700 rat genes. Using the criterion of fold change > 4 in the expression level, we identified 83 genes that were up-regulated and 89 genes that were down-regulated in oligodendrocytes, and 92 genes that were up-regulated and 86 that were down-regulated in type II astrocytes compared with OPCs. -

Thyroglobulin (Tg) Assays
Thyroglobulin (Tg) Assays ABOUT DTC patients is limited. In the US, a Tg-RIA with a functional sensitivity (9, 10) The Laboratory Services Committee of the American Thyroid of 0.5 µg/L is available for clinical purposes. Although the functional Association® (ATA) conducted a survey of ATA® members to identify sensitivity of this assay is still suboptimal, it has been extensively used as areas of member interest for education in pathology and laboratory a “gold standard” in studies evaluating the effect of TgAb interference in (4, 11, 12, 13) medicine. In response to the results of the survey, the Lab Service Tg IMA. This assay is promoted as being more resistant to TgAb Committee developed a series of educational materials to share with the interferences due to the use of polyclonal antibodies that could recognize 9 ATA® membership. The topics below were ranked as high educational Tg epitopes even when bound to TgAb. However, interferences in Tg-RIA priorities amongst the membership. results have been reported. Early studies reported that TgAb caused an overestimation of Tg by RIA.14 Others have reported underestimation of MEASUREMENT OF SERUM THYROGLOBULIN Tg by RIA in the presence of TgAb.(15,16) Thyroglobulin (Tg) measurement is an integral part of the follow up IMMUNOMETRIC ASSAYS and management of patients with differentiated thyroid cancer (DTC). Tg-IMA are based on a two-site reaction that involves Tg capture by Although, measurement of Tg for initial evaluation of suspicious thyroid a solid-phase antibody followed by addition of a labeled antibody that nodules is not recommended, serum Tg measurements are used targets different epitopes on the captured Tg. -

A Study on a Large Cohort of Renal Transplant Patients This Information Is Current As of September 24, 2021
Generation of Catalytic Antibodies Is an Intrinsic Property of an Individual's Immune System: A Study on a Large Cohort of Renal Transplant Patients This information is current as of September 24, 2021. Ankit Mahendra, Ivan Peyron, Olivier Thaunat, Cécile Dollinger, Laurent Gilardin, Meenu Sharma, Bharath Wootla, Desirazu N. Rao, Séverine Padiolleau-Lefevre, Didier Boquet, Abhijit More, Navin Varadarajan, Srini V. Kaveri, Christophe Legendre and Sébastien Lacroix-Desmazes Downloaded from J Immunol 2016; 196:4075-4081; Prepublished online 11 April 2016; doi: 10.4049/jimmunol.1403005 http://www.jimmunol.org/content/196/10/4075 http://www.jimmunol.org/ Supplementary http://www.jimmunol.org/content/suppl/2016/04/09/jimmunol.140300 Material 5.DCSupplemental References This article cites 20 articles, 12 of which you can access for free at: http://www.jimmunol.org/content/196/10/4075.full#ref-list-1 by guest on September 24, 2021 Why The JI? Submit online. • Rapid Reviews! 30 days* from submission to initial decision • No Triage! Every submission reviewed by practicing scientists • Fast Publication! 4 weeks from acceptance to publication *average Subscription Information about subscribing to The Journal of Immunology is online at: http://jimmunol.org/subscription Permissions Submit copyright permission requests at: http://www.aai.org/About/Publications/JI/copyright.html Email Alerts Receive free email-alerts when new articles cite this article. Sign up at: http://jimmunol.org/alerts The Journal of Immunology is published twice each month by The American Association of Immunologists, Inc., 1451 Rockville Pike, Suite 650, Rockville, MD 20852 Copyright © 2016 by The American Association of Immunologists, Inc. -

Partial Purification and Properties of a Plasminogen Activator from Human Erythrocytes
Partial purification and properties of a plasminogen activator from human erythrocytes M. Semar, … , L. Skoza, A. J. Johnson J Clin Invest. 1969;48(10):1777-1785. https://doi.org/10.1172/JCI106144. Research Article The lysis time of euglobulin clots made with whole blood (plasma and red cells) was very much shorter than that of clots made with plasma alone, indicating a fibrinolytic component in red cells. A plasminogen activator was found in the stroma-free hemolysate, and proteolytic activity was found in the stromal fraction. The plasminogen activator, purified by using diethylaminoethyl-cellulose (DEAE-cellulose) in a batch procedure followed by column chromatography, was called erythrokinase (EK). On preliminary characterization, EK appears to activate human and bovine plasminogen in a manner similar to urokinase (UK), as determined by fibrinolytic and caseinolytic assays. The two enzymes can be separated by DEAE chromatography and acrylamide-gel electrophoresis, however, and they hydrolyze acetyl-L-lysine methyl ester and benzoyl arginine methyl ester at different rates. Find the latest version: https://jci.me/106144/pdf Partial Purification and Properties of a Plasminogen Activator from Human Erythrocytes M. SEMAR, L. SKOZA, and A. J. JOHNSON From the Department of Medicine, New York University Medical Center, and the American National Red Cross Research Laboratory, New York 10016 A B S T R A C T The lysis time of euglobulin clots made research on the fibrinolytic components contained in with whole blood (plasma and red cells) was very much the red cell, or on the possible physiologic role of red shorter than that of clots made with plasma alone, in- cells in thrombolysis. -

Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients
diseases Article Severity of Anaemia Has Corresponding Effects on Coagulation Parameters of Sickle Cell Disease Patients Samuel Antwi-Baffour 1,* , Ransford Kyeremeh 1 and Lawrence Annison 2 1 Department of Medical Laboratory Sciences, School of Allied Health Sciences, College of Health Sciences, University of Ghana, P.O. Box KB 143 Accra, Ghana; [email protected] 2 Department of Medical Laboratory Sciences, School of Allied Health Sciences, Narh-Bita College, P.O. Box Co1061 Tema, Ghana; [email protected] * Correspondence: s.antwi-baff[email protected] Received: 31 July 2019; Accepted: 18 October 2019; Published: 17 December 2019 Abstract: Sickle cell disease (SCD) is an inherited condition characterized by chronic haemolytic anaemia. SCD is associated with moderate to severe anaemia, hypercoagulable state and inconsistent platelet count and function. However, studies have yielded conflicting results with regards to the effect of anaemia on coagulation in SCD. The purpose of this study was to determine the effect of anaemia severity on selected coagulation parameters of SCD patients. Four millilitres of venous blood samples were taken from the participants (SCD and non-SCD patients) and used for analysis of full blood count and coagulation parameters. Data was analysed using SPSS version-16. From the results, it was seen that individuals with SCD had a prolonged mean PT, APTT and high platelet count compared to the controls. There was also significant difference in the mean PT (p = 0.039), APTT (p = 0.041) and platelet count (p = 0.010) in HbSS participants with severe anaemia. Mean APTT also showed significant difference (p = 0.044) with severe anaemia in HbSC participants. -

Sudan University of Science and Technology College of Medical Laboratory Science Department of Hematology
بسم هللا الرحمن الرحيم Sudan University of Science and Technology College of Medical Laboratory Science Department of Hematology D-dimer, PT and APTT levels among Renal Transplant Patients in Sudan مستويات الدي دايمر ,زمن البروثرومبين و زمن الثرموبوبﻻستين الجزئي المنشط وسط مرضي زراعة الكلى في السودان A thesis submitted for partial fulfillment of requirements of M.Sc. degree in Hematology Student Zubaida Mohamed Ahmed Abdalbagi, B.S.c 2013 Department of Hematology – Faculty of Medical Laboratory Sciences - Khartoum University Supervisor Dr. Hiba Badreldin Khalil, PhD Department of Hematology – Faculty of Medical Laboratory Sciences - Alneelain University 2019 بسم هللا الرحمن الرحيم قال تعالى : ْ اقْرَأْْبِاسْمِْْرَ بِكَْْاَّلِذيْخََلقَْ سورةْالعلقْ صدق هللا العظيمْ List of Contents Contents Page No I اﻵية List of Contents II List of Figures VI List of Tables VII List of Abbreviations VIII Dedication XI Acknowledgement XII Abstract / English Abstract XIII Arabic Abstract XIV / ملخص الدراسة Chapter One 1.1 Chronic Kidney Disease 1 1.1.1 Causes of Chronic Kidney Disease 6 1.1.2 Diagnosis 7 1.1.2.1 Differential diagnosis 7 1.1.3 Severity-Based Stages 7 1.1.4 Treatment of Chronic Kidney Disease 9 1.1.5 Prognosis of Chronic Kidney Disease 10 1.2 Kidney Transplantation 11 1.2.1 History of Kidney Transplantation 11 1.2.2 Indications 13 1.2.3.1Living donors 13 1.2.4 Deceased donors 61 1.2.5 Compatibility 18 1.2.6 Procedure 19 1.2.7 Post Operation 20 1.2.8 Complications 26 1.2.9 Prognosis 22 1.3 Homeostasis and Coagulation 23 1.3.1Nomenclature 24 -

Blood and Immunity
Chapter Ten BLOOD AND IMMUNITY Chapter Contents 10 Pretest Clinical Aspects of Immunity Blood Chapter Review Immunity Case Studies Word Parts Pertaining to Blood and Immunity Crossword Puzzle Clinical Aspects of Blood Objectives After study of this chapter you should be able to: 1. Describe the composition of the blood plasma. 7. Identify and use roots pertaining to blood 2. Describe and give the functions of the three types of chemistry. blood cells. 8. List and describe the major disorders of the blood. 3. Label pictures of the blood cells. 9. List and describe the major disorders of the 4. Explain the basis of blood types. immune system. 5. Define immunity and list the possible sources of 10. Describe the major tests used to study blood. immunity. 11. Interpret abbreviations used in blood studies. 6. Identify and use roots and suffixes pertaining to the 12. Analyse several case studies involving the blood. blood and immunity. Pretest 1. The scientific name for red blood cells 5. Substances produced by immune cells that is . counteract microorganisms and other foreign 2. The scientific name for white blood cells materials are called . is . 6. A deficiency of hemoglobin results in the disorder 3. Platelets, or thrombocytes, are involved in called . 7. A neoplasm involving overgrowth of white blood 4. The white blood cells active in adaptive immunity cells is called . are the . 225 226 ♦ PART THREE / Body Systems Other 1% Proteins 8% Plasma 55% Water 91% Whole blood Leukocytes and platelets Formed 0.9% elements 45% Erythrocytes 10 99.1% Figure 10-1 Composition of whole blood.