Microbial Synthesis of Unique Nanoscale Minerals - Challenges and Prospects
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

The Diatoms Big Significance of Tiny Glass Houses
GENERAL ¨ ARTICLE The Diatoms Big Significance of Tiny Glass Houses Aditi Kale and Balasubramanian Karthick Diatoms are unique microscopic algae having intricate cell walls made up of silica. They are the major phytoplankton in aquatic ecosystems and account for 20–25% of the oxygen release and carbon fixation in the world. Their most charac- teristic features are mechanisms they have evolved to utilize silica. Due to their distinctive adaptations and ecology, they (left) Aditi Kale is a PhD student with the are used in various fields like biomonitoring, paleoecology, Biodiversity and nanotechnology and forensics. Paleobiology group of Agharkar Research Introduction Institute. She is studying the biogeography of Diatoms (Class: Bacillariophyceae) are unique microscopic al- freshwater diatoms in gae containing silica and having distinct geometrical shapes. Western Ghats for her They are unicellular, eukaryotic and photosynthetic organisms. thesis. Their cell size ranges between 5 µm–0.5 mm. They occur in wet (right) Balasubramanian or moist places where photosynthesis is possible. Diatoms are Karthick is Scientist with Biodiversity and either planktonic (free-floating) or benthic (attached to a substra- Paleobiology group of tum) in Nature (Figure 1). The individuals are solitary or some- Agharkar Research times form colonies. Diatoms are mostly non-motile; however, Institute. His interests some benthic diatoms have a specialized raphe system1 that include diatom taxonomy and ecology, microbial secretes mucilage to attach or glide along a surface. They are also biogeography,andaquatic known to form biofilms, i. e., layers of tightly attached cells of ecology. microorganisms. Biofilms are formed on a solid surface and are often surrounded by extra-cellular fluids. -

Magnetotactic Bacteria and Their Application in Medicine
Chem cal ist si ry y & h P B f i o o Dasdag and Bektas. J Phys Chem Biophys 2014, 4:2 p l h a Journal of Physical Chemistry & y n s r DOI: 10.4172/2161-0398.1000141 i u c o s J ISSN: 2161-0398 Biophysics ResearchReview Article Article OpenOpen Access Access Magnetotactic Bacteria and their Application in Medicine Suleyman Dasdag1* and Hava Bektas2 1Department of Biophysics, Medical School of Dicle University, Diyarbakir, Turkey 2Department of Biophysics, Medical School of Yuzuncu Yil University, Van / Turkey Abstract It is a known fact how the magnetic field of the Earth is very important for life. Relation between living systems and the earth magnetic field has been investigated for many years. Birds and their migration routes are the first one of the things that comes to mind when we state living things. The Earth’s magnetic field is still accepted to be the main factor for birds and other flying living beings to complete their travels correctly. The changes in migration routes, which are observed from time to time, are sometimes said to be due to the changes in the magnetic field. However, no light has been shed to this matter yet. The Earth’s magnetic field has not been sufficiently studied, and its role on small living models such as bacteria has not been adequately discussed. One of the best examples in this field is relation between the Earth’s magnetic field and “magnetotactic bacteria (MTB)”, which were discovered by Salvatore Bellini in 1963. Currently, it is claimed that magnetotactic bacteria have a widespread use in microbiology, mineralogy, limnology, physics, biophysics, chemistry, biochemistry, geology, crystallography, and astrobiology. -

Life with Compass: Diversity and Biogeography of Magnetotactic Bacteria
bs_bs_banner Environmental Microbiology (2014) 16(9), 2646–2658 doi:10.1111/1462-2920.12313 Minireview Life with compass: diversity and biogeography of magnetotactic bacteria Wei Lin,1,2 Dennis A. Bazylinski,3 Tian Xiao,2,4 the present-day biogeography of MTB, and the ruling Long-Fei Wu2,5 and Yongxin Pan1,2* parameters of their spatial distribution, will eventu- 1Biogeomagnetism Group, Paleomagnetism and ally help us predict MTB community shifts with envi- Geochronology Laboratory, Key Laboratory of the ronmental changes and assess their roles in global Earth’s Deep Interior, Institute of Geology and iron cycling. Geophysics, Chinese Academy of Sciences, Beijing 100029, China. 2France-China Bio-Mineralization and Nano-Structures Introduction Laboratory, Chinese Academy of Sciences, Beijing Iron is the fourth most common element in the Earth’s 100029, China. crust and a crucial nutrient for almost all known organ- 3 School of Life Sciences, University of Nevada at Las isms. The cycling of iron is one of the key processes in the Vegas, Las Vegas, NV, USA. Earth’s biogeochemical cycles. A number of organisms 4 Key Laboratory of Marine Ecology & Environmental synthesize iron minerals and play essential roles in global Sciences, Institute of Oceanology, Chinese Academy of iron cycling (Westbroek and de Jong, 1983; Winklhofer, Sciences, Qingdao, China. 2010). One of the most interesting examples of these 5 Laboratoire de Chimie Bactérienne, Aix-Marseille types of organisms are the magnetotactic bacteria (MTB), Université, CNRS, Marseille Cedex, France. a polyphyletic group of prokaryotes that are ubiquitous in aquatic and sedimentary environments (Bazylinski Summary and Frankel, 2004; Bazylinski et al., 2013). -

Overcalcified Forms of the Coccolithophore Emiliania Huxleyi in High CO2 Waters Are Not Pre-Adapted to Ocean Acidification
Biogeosciences Discuss., https://doi.org/10.5194/bg-2017-303 Manuscript under review for journal Biogeosciences Discussion started: 6 September 2017 c Author(s) 2017. CC BY 4.0 License. Overcalcified forms of the coccolithophore Emiliania huxleyi in high CO2 waters are not pre-adapted to ocean acidification. Peter von Dassow1,2,3*, Francisco Díaz-Rosas1,2, El Mahdi Bendif4, Juan-Diego Gaitán-Espitia5, Daniella Mella-Flores1, Sebastian Rokitta6, Uwe John6, and Rodrigo Torres7,8 5 1 Facultad de Ciencias Biológicas, Pontificia Universidad Católica de Chile, Santiago, Chile. 2 Instituto Milenio de Oceanografía de Chile. 3 UMI 3614, Evolutionary Biology and Ecology of Algae, CNRS-UPMC Sorbonne Universités, PUCCh, UACH. 4 Department of Plant Sciences, University of Oxford, OX1 3RB Oxford, UK. 5 CSIRO Oceans and Atmosphere, GPO Box 1538, Hobart 7001, TAS, Australia. 10 6 Marine Biogeosciences | PhytoChange Alfred Wegener Institute – Helmholtz Centre for Polar and Marine Research, Bremerhaven, Germany. 7 Centro de Investigación en Ecosistemas de la Patagonia (CIEP), Coyhaique, Chile. 8 Centro de Investigación: Dinámica de Ecosistemas marinos de Altas Latitudes (IDEAL), Punta Arenas, Chile. Correspondence to: Peter von Dassow ([email protected]) 15 Abstract. Marine multicellular organisms inhabiting waters with natural high fluctuations in pH appear more tolerant to acidification than conspecifics occurring in nearby stable waters, suggesting that environments of fluctuating pH hold genetic reservoirs for adaptation of key groups to ocean acidification (OA). The abundant and cosmopolitan calcifying phytoplankton Emiliania huxleyi exhibits a range of morphotypes with varying degrees of coccolith mineralization. We show that E. huxleyi populations in the naturally acidified upwelling waters of the Eastern South Pacific, where pH drops below 20 7.8 as is predicted for the global surface ocean by the year 2100, are dominated by exceptionally overcalcified morphotypes whose distal coccolith shield can be almost solid calcite. -

Phytoplankton As Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate
sustainability Review Phytoplankton as Key Mediators of the Biological Carbon Pump: Their Responses to a Changing Climate Samarpita Basu * ID and Katherine R. M. Mackey Earth System Science, University of California Irvine, Irvine, CA 92697, USA; [email protected] * Correspondence: [email protected] Received: 7 January 2018; Accepted: 12 March 2018; Published: 19 March 2018 Abstract: The world’s oceans are a major sink for atmospheric carbon dioxide (CO2). The biological carbon pump plays a vital role in the net transfer of CO2 from the atmosphere to the oceans and then to the sediments, subsequently maintaining atmospheric CO2 at significantly lower levels than would be the case if it did not exist. The efficiency of the biological pump is a function of phytoplankton physiology and community structure, which are in turn governed by the physical and chemical conditions of the ocean. However, only a few studies have focused on the importance of phytoplankton community structure to the biological pump. Because global change is expected to influence carbon and nutrient availability, temperature and light (via stratification), an improved understanding of how phytoplankton community size structure will respond in the future is required to gain insight into the biological pump and the ability of the ocean to act as a long-term sink for atmospheric CO2. This review article aims to explore the potential impacts of predicted changes in global temperature and the carbonate system on phytoplankton cell size, species and elemental composition, so as to shed light on the ability of the biological pump to sequester carbon in the future ocean. -
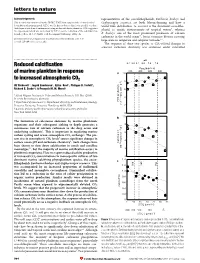
Reduced Calcification of Marine Plankton in Response to Increased
letters to nature Acknowledgements representatives of the coccolithophorids, Emiliania huxleyi and This research was sponsored by the EPSRC. T.W.F. ®rst suggested the electrochemical Gephyrocapsa oceanica, are both bloom-forming and have a deoxidation of titanium metal. G.Z.C. was the ®rst to observe that it was possible to reduce world-wide distribution. G. oceanica is the dominant coccolitho- thick layers of oxide on titanium metal using molten salt electrochemistry. D.J.F. suggested phorid in neritic environments of tropical waters9, whereas the experiment, which was carried out by G.Z.C., on the reduction of the solid titanium dioxide pellets. M. S. P. Shaffer took the original SEM image of Fig. 4a. E. huxleyi, one of the most prominent producers of calcium carbonate in the world ocean10, forms extensive blooms covering Correspondence and requests for materials should be addressed to D. J. F. large areas in temperate and subpolar latitudes9,11. (e-mail: [email protected]). The response of these two species to CO2-related changes in seawater carbonate chemistry was examined under controlled ................................................................. pH Reduced calci®cation 8.4 8.2 8.1 8.0 7.9 7.8 PCO2 (p.p.m.v.) of marine plankton in response 200 400 600 800 a 10 to increased atmospheric CO2 ) 8 –1 Ulf Riebesell *, Ingrid Zondervan*, BjoÈrn Rost*, Philippe D. Tortell², d –1 Richard E. Zeebe*³ & FrancËois M. M. Morel² 6 * Alfred Wegener Institute for Polar and Marine Research, P.O. Box 120161, 4 D-27515 Bremerhaven, Germany mol C cell –13 ² Department of Geosciences & Department of Ecology and Evolutionary Biology, POC production Princeton University, Princeton, New Jersey 08544, USA (10 2 ³ Lamont-Doherty Earth Observatory, Columbia University, Palisades, New York 10964, USA 0 ............................................................................................................................................. -

Investigation of the Magnetosome Biomineralization in Magnetotactic Bacteria Using Graphene Liquid Cell – Transmission Electron Microscopy
Nanoscale Investigation of the Magnetosome Biomineralization in Magnetotactic Bacteria using Graphene Liquid Cell – Transmission Electron Microscopy Journal: Nanoscale Manuscript ID NR-ART-10-2018-008647.R1 Article Type: Paper Date Submitted by the 27-Nov-2018 Author: Complete List of Authors: Firlar, Emre; University of Illinois at Chicago, Department of Bioengineering; University of Illinois at Chicago, Department of Mechanical and Industrial Engineering Ouy, Meagan; University of Illinois at Chicago, Department of Bioengineering Bogdanowicz, Agata; University of Illinois at Chicago, Department of Bioengineering Covnot, Leigha; University of Illinois at Chicago, Department of Bioengineering Song, Boao; University of Illinois at Chicago, Mechanical Engineering Department Nadkarni, Yash; University of Illinois at Chicago, Department of Bioengineering Shahbazian-Yassar, Reza; University of Illinois at Chicago, Mechanical and Industrial Engineering; University of Illinois at Chicago Shokuhfar, Tolou; University of Illinois at Chicago, Department of Bioengineering; Michigan Technological University, Department of Mechanical Engineering Page 1 of 8 Please doNanoscale not adjust margins Journal Name ARTICLE Investigation of the Magnetosome Biomineralization in Magnetotactic Bacteria using Graphene Liquid Cell – Transmission Electron Microscopy Received 00th January 20xx, Accepted 00th January 20xx Emre Firlara,b*, Meagan Ouya, Agata Bogdanowicza, Leigha Covnota, Boao Songb, Yash Nadkarnia, DOI: 10.1039/x0xx00000x Reza Shahbazian-Yassarb*, -

Stromatolites Below the Photic Zone in the Northern Arabian Sea Formed by Calcifying Chemotrophic Microbial Mats
Stromatolites below the photic zone in the northern Arabian Sea formed by calcifying chemotrophic microbial mats Tobias Himmler1*, Daniel Smrzka2, Jennifer Zwicker2, Sabine Kasten3,4, Russell S. Shapiro5, Gerhard Bohrmann1, and Jörn Peckmann2,6 1MARUM–Zentrum für Marine Umweltwissenschaften und Fachbereich Geowissenschaften, Universität Bremen, 28334 Bremen, Germany 2Department für Geodynamik und Sedimentologie, Erdwissenschaftliches Zentrum, Universität Wien, 1090 Wien, Austria 3Alfred-Wegener-Institut Helmholtz-Zentrum für Polar- und Meeresforschung, 27570 Bremerhaven, Germany 4Fachbereich Geowissenschaften, Universität Bremen, 28359 Bremen, Germany 5Geological and Environmental Sciences Department, California State University–Chico, Chico, California 95929, USA 6Institut für Geologie, Centrum für Erdsystemforschung und Nachhaltigkeit, Universität Hamburg, 20146 Hamburg, Germany − − + 2− + ABSTRACT reduction: HS + NO3 + H + H2O → SO4 + NH4 (cf. Fossing et al., 1995; Chemosynthesis increases alkalinity and facilitates stromatolite Otte et al., 1999). The effect of this process, referred to as nitrate-driven growth at methane seeps in 731 m water depth within the oxygen sulfide oxidation (ND-SO), is amplified when it takes place near hotspots minimum zone (OMZ) in the northern Arabian Sea. Microbial fab- of SD-AOM (Siegert et al., 2013). Therefore, it has been hypothesized rics, including mineralized filament bundles resembling the sulfide- that fossilization of sulfide-oxidizing bacteria may occur during seep- oxidizing bacterium Thioploca, mineralized extracellular polymeric carbonate formation (Bailey et al., 2009). Yet, seep carbonates resulting substances, and fossilized rod-shaped and filamentous cells, all pre- from the putative interaction of sulfide-oxidizing bacteria with the SD- served in 13C-depleted authigenic carbonate, suggest that biofilm cal- AOM consortium have, to the best of our knowledge, only been recognized cification resulted from nitrate-driven sulfide oxidation (ND-SO) and in ancient seep deposits (Peckmann et al., 2004). -

Magnetic Properties of Uncultivated Magnetotactic Bacteria and Their Contribution to a Stratified Estuary Iron Cycle
ARTICLE Received 6 Feb 2014 | Accepted 25 Jul 2014 | Published 1 Sep 2014 DOI: 10.1038/ncomms5797 Magnetic properties of uncultivated magnetotactic bacteria and their contribution to a stratified estuary iron cycle A.P. Chen1, V.M. Berounsky2, M.K. Chan3, M.G. Blackford4, C. Cady5,w, B.M. Moskowitz6, P. Kraal7, E.A. Lima8, R.E. Kopp9, G.R. Lumpkin4, B.P. Weiss8, P. Hesse1 & N.G.F. Vella10 Of the two nanocrystal (magnetosome) compositions biosynthesized by magnetotactic bacteria (MTB), the magnetic properties of magnetite magnetosomes have been extensively studied using widely available cultures, while those of greigite magnetosomes remain poorly known. Here we have collected uncultivated magnetite- and greigite-producing MTB to determine their magnetic coercivity distribution and ferromagnetic resonance (FMR) spectra and to assess the MTB-associated iron flux. We find that compared with magnetite-producing MTB cultures, FMR spectra of uncultivated MTB are characterized by a wider empirical parameter range, thus complicating the use of FMR for fossilized magnetosome (magnetofossil) detection. Furthermore, in stark contrast to putative Neogene greigite magnetofossil records, the coercivity distributions for greigite-producing MTB are fundamentally left-skewed with a lower median. Lastly, a comparison between the MTB-associated iron flux in the investigated estuary and the pyritic-Fe flux in the Black Sea suggests MTB play an important, but heretofore overlooked role in euxinic marine system iron cycle. 1 Department of Environment and Geography, Macquarie University, North Ryde, New South Wales 2109, Australia. 2 Graduate School of Oceanography, University of Rhode Island, Narragansett, Rhode Island 02882, USA. 3 School of Physics and Astronomy, University of Minnesota, Minneapolis, Minnesota 55455, USA. -

A Perspective on Underlying Crystal Growth Mechanisms in Biomineralization: Solution Mediated Growth Versus Nanosphere Particle Accretion
CrystEngComm A perspective on underlying crystal growth mechanisms in biomineralization: solution mediated growth versus nanosphere particle accretion Journal: CrystEngComm Manuscript ID: CE-HIG-07-2014-001474.R1 Article Type: Highlight Date Submitted by the Author: 01-Dec-2014 Complete List of Authors: Gal, Assaf; Weizmann Institute of Science, Structural Biology Weiner, Steve; Weizmann Institute of Science, Structural Biology Addadi, Lia; Weizmann Institute of Science, Structural Biology Page 1 of 23 CrystEngComm A perspective on underlying crystal growth mechanisms in biomineralization: solution mediated growth versus nanosphere particle accretion Assaf Gal, Steve Weiner, and Lia Addadi Department of Structural Biology, Weizmann Institute of Science, Rehovot, Israel 76100 Abstract Many organisms form crystals from transient amorphous precursor phases. In the cases where the precursor phases were imaged, they consist of nanosphere particles. Interestingly, some mature biogenic crystals also have nanosphere particle morphology, but some are characterized by crystallographic faces that are smooth at the nanometer level. There are also biogenic crystals that have both crystallographic faces and nanosphere particle morphology. This highlight presents a working hypothesis, stating that some biomineralization processes involve growth by nanosphere particle accretion, where amorphous nanoparticles are incorporated as such into growing crystals and preserve their morphology upon crystallization. This process produces biogenic crystals with a nanosphere particle morphology. Other biomineralization processes proceed by ion-by-ion growth, and some cases of biological crystal growth involve both processes. We also identify several biomineralization processes which do not seem to fit this working hypothesis. It is our hope that this highlight will inspire studies that will shed more light on the underlying crystallization mechanisms in biology. -

Historical Painting Techniques, Materials, and Studio Practice
Historical Painting Techniques, Materials, and Studio Practice PUBLICATIONS COORDINATION: Dinah Berland EDITING & PRODUCTION COORDINATION: Corinne Lightweaver EDITORIAL CONSULTATION: Jo Hill COVER DESIGN: Jackie Gallagher-Lange PRODUCTION & PRINTING: Allen Press, Inc., Lawrence, Kansas SYMPOSIUM ORGANIZERS: Erma Hermens, Art History Institute of the University of Leiden Marja Peek, Central Research Laboratory for Objects of Art and Science, Amsterdam © 1995 by The J. Paul Getty Trust All rights reserved Printed in the United States of America ISBN 0-89236-322-3 The Getty Conservation Institute is committed to the preservation of cultural heritage worldwide. The Institute seeks to advance scientiRc knowledge and professional practice and to raise public awareness of conservation. Through research, training, documentation, exchange of information, and ReId projects, the Institute addresses issues related to the conservation of museum objects and archival collections, archaeological monuments and sites, and historic bUildings and cities. The Institute is an operating program of the J. Paul Getty Trust. COVER ILLUSTRATION Gherardo Cibo, "Colchico," folio 17r of Herbarium, ca. 1570. Courtesy of the British Library. FRONTISPIECE Detail from Jan Baptiste Collaert, Color Olivi, 1566-1628. After Johannes Stradanus. Courtesy of the Rijksmuseum-Stichting, Amsterdam. Library of Congress Cataloguing-in-Publication Data Historical painting techniques, materials, and studio practice : preprints of a symposium [held at] University of Leiden, the Netherlands, 26-29 June 1995/ edited by Arie Wallert, Erma Hermens, and Marja Peek. p. cm. Includes bibliographical references. ISBN 0-89236-322-3 (pbk.) 1. Painting-Techniques-Congresses. 2. Artists' materials- -Congresses. 3. Polychromy-Congresses. I. Wallert, Arie, 1950- II. Hermens, Erma, 1958- . III. Peek, Marja, 1961- ND1500.H57 1995 751' .09-dc20 95-9805 CIP Second printing 1996 iv Contents vii Foreword viii Preface 1 Leslie A. -

Synthetic Morphogenesis
Downloaded from http://cshperspectives.cshlp.org/ on September 24, 2021 - Published by Cold Spring Harbor Laboratory Press Synthetic Morphogenesis Brian P. Teague, Patrick Guye, and Ron Weiss Synthetic Biology Center, Department of Biological Engineering, Massachusetts Institute of Technology, Cambridge, Massachusetts 02139 Correspondence: [email protected] Throughout biology, function is intimately linked with form. Across scales ranging from subcellular to multiorganismal, the identity and organization of a biological structure’s subunits dictate its properties. The field of molecular morphogenesis has traditionally been concerned with describing these links, decoding the molecular mechanisms that give rise to the shape and structure of cells, tissues, organs, and organisms. Recent advances in synthetic biology promise unprecedented control over these molecular mechanisms; this opens the path to not just probing morphogenesis but directing it. This review explores several frontiers in the nascent field of synthetic morphogenesis, including programmable tissues and organs, synthetic biomaterials and programmable matter, and engineering complex morphogenic systems de novo. We will discuss each frontier’s objectives, current approaches, constraints and challenges, and future potential. hat is the underlying basis of biological 2014). As the mechanistic underpinnings of Wstructure? Speculations were based on these processes are elucidated, opportunities macroscopic observation until the 17th century, arise to use this knowledge to direct mor- when Antonie van Leeuwenhoek and Robert phogenesis toward novel, useful ends. We call Hooke discovered that organisms were com- this emerging field of endeavor “synthetic mor- posed of microscopic cells (Harris 1999), and phogenesis.” Inspired by and based on natural that the size and shape of the cells affected the morphogenic systems, synthetic morphogenesis properties of the structures they formed.