Pakej Bantuan Rakyat Pulau Pinang
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Penang Page 1 Area Location State Outskirt ODA 10990 Penang Yes
Penang Post Major code Area Location State Town Outskirt ODA Delivery Day Delivery Delivery Day - 1 to 2 Day - 1 to 7 - 3 to 4 working working working days days days 10990 Pulau Pinang - Beg berkunci Pulau Pinang Penang Yes 11000 Focus Heights Balik Pulau Penang Yes 11000 Jalan Pinang Nirai Balik Pulau Penang Yes 11000 Kampung Kuala Muda Balik Pulau Penang Yes 11000 Kebun Besar Balik Pulau Penang Yes 11000 Kuala Muda Balik Pulau Penang Yes 11000 Padang Kemunting Mk. E Balik Pulau Penang Yes 11000 Padang Kemunting Balik Pulau Penang Yes 10000 Bangunan Komtar Pulau Pinang Penang Yes 10000 Jalan Gladstone Pulau Pinang Penang Yes 10000 Jalan Magazine (No Genap) Pulau Pinang Penang Yes 10000 Kompleks Tun Abdul Razak Pulau Pinang Penang Yes 10000 Lebuh Tek Soon Pulau Pinang Penang Yes 10000 Prangin Mall Pulau Pinang Penang Yes 10050 Jalan Argyll Pulau Pinang Penang Yes 10050 Jalan Ariffin Pulau Pinang Penang Yes 10050 Jalan Arratoon Pulau Pinang Penang Yes 10050 Jalan Bawasah Pulau Pinang Penang Yes 10050 Jalan Burma (1 - 237 & 2 - 184) Pulau Pinang Penang Yes 10050 Jalan Chow Thye Pulau Pinang Penang Yes 10050 Jalan Clove Hall Pulau Pinang Penang Yes 10050 Jalan Dato Koyah Pulau Pinang Penang Yes 10050 Jalan Dinding Pulau Pinang Penang Yes 10050 Jalan Gudwara Pulau Pinang Penang Yes 10050 Jalan Hutton Pulau Pinang Penang Yes 10050 Jalan Irawadi Pulau Pinang Penang Yes 10050 Jalan Khoo Sian Ewe Pulau Pinang Penang Yes 10050 Jalan Larut Pulau Pinang Penang Yes 10050 Jalan Nagore Pulau Pinang Penang Yes 10050 Jalan Pangkor Pulau Pinang Penang -

BKT DUMBAR NEWS.Pages
18/9/2016 OFFICIAL LAUNCHING OF BUKIT DUMBAR PUMPING STATION 2 Community Home > Metro > Community Tuesday, 20 September 2016 Southern Penang gets uninterrupted water supply CONTINUOUS good water supply to the Bayan Lepas Free Trade Zone, Penang International Airport and southern parts of Penang island is now better guaranteed following the commission of a new water pump station at Bukit Dumbar. Called BD2, it could pump up to 270 million litres of water per day (MLD) to serve 315,000 people living in the southern parts of the island. PBAPP senior chargeman Mohd Yusri Awang checking the reading of a pump at the newly opened Bukit Dumbar Pump Station 2 in Penang. Its service areas cover Gelugor, Batu Uban, Sungai Nibong, Bayan Baru, Relau, Sungai Ara, Batu Maung, Bayan Lepas, Permatang Damar Laut, Teluk Kumbar, Gertak Sanggul, Genting and Balik Pulau. Penang Water Supply Corporation Sdn Bhd (PBAPP) chief executive officer Datuk Jaseni Maidinsa said the RM11.9mil BD2 would complement the operations of the Bukit Dumbar Pump Station 1 (BD1) that had been in service since 1980. He said it would improve pumping efficiency of water from the Sungai Dua Water Treatment Plant on the mainland to southern areas of the island which were undergoing rapid socio-economic development. “Treated water from the Sungai Dua plant is delivered to Bukit Dumbar daily via twin submarine pipeline,” Jaseni said at the launching of BD2 on Sunday. He said BD2 would also reduce pumping costs to the Bukit Gedong Reservoir daily to support the treated water needs of Teluk Kumbar, Gertak Sanggul and Balik Pulau. -

ZON BATU MAUNG Nama Syarikat ERA BUMIWAY SDN. BHD
KUTIPAN SAMPAH PUKAL DAN KEBUN ZON BATU MAUNG Nama Syarikat ERA BUMIWAY SDN. BHD. G10 SAMPAH KEBUN SAMPAH PUKAL TRIP NAMA JALAN NAMA TAMAN NAMA KAMPUNG HARI WAKTU HARI WAKTU LENGKOK BATU MAUNG 3 KAWASAN PERUMAHAN TAMAN IPING LORONG TELUK TEMPOYAK 1-3 LORONG BATU NILAM 1-6 PERUMAHAN BATU NILAM JALAN BIDARA 1-5 PERUMAHAN JALAN BIDARA JALAN JELITI 1 PERUMAHAN JALAN JELITI LORONG JELITI 2 LORONG JELITI 1 135 7:00-10:00 PAGI 246 7:00-10:00 PAGI 1 JALAN JELITI JALAN KEKABU LORONG KEKABU SOLOK KEKABU SEK KEB PERMATANG DAMAR LAUT JLN KEKABU 1-8 PERUMAHAN JALAN KEKABU LORONG KEKABU 1-2 PERMATANG DAMAR LAUT ZON 8 KG CINA PERMATANG DAMAR LAUT PERMATANG DAMAR LAUT ZON 6 KG PERMATANG DAMAR LAUT LINTANG DAMAR LAUT KG DAMAR LAUT 135 10:00-12:00 T/HARI 246 10:00-12:00 T/HARI 2 LINTANG DAMAR LAUT 1-7 PERUMAHAN JALAN DAMAR MEDAN BATU MAUNG 1-8 TAMAN BATU MAUNG KG NARAN TAMAN IPING KG NARAN Astaka Seagate Restorent Telok Tempoyak Perkampungan Telok Tempoyak KG TELUK TEMPOYAK Kepok Teluk Tempoyak Sek Keb Batu Maung Rumah Kedai 2 Tingkat, Lebuh raya Batu Maung Block 71, Desa Mutiara Indah Block 73, Desa Mutiara Indah 3 135 12:00-2:00 PETANG 246 12:00-2:00 PETANG Lengkok Batu Maung 1 Flat Muhibbah, Lengkok Batu Maung 1 Flat Taman Indah,Fasa 4 3 Flat Taman Indah,Fasa 7 135 12:00-2:00 PETANG 246 12:00-2:00 PETANG Block 34/36 Taman Mewah Block 54/57 Taman Mewah Main Road Batu Maung SJKK Weng Kai Astaka dan Pasar Batu Maung Mukim 12 Jln Batu Maung Astaka Lintang Bayan Lepas 2 Lintang Beringin 1-11 Hala beringin Jln Beringin Lintang Beingin 6 Rumah Kedai 2 Tingkat -
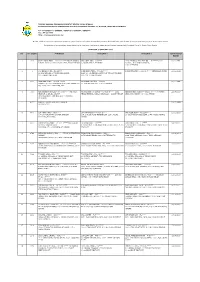
Fax : 04-2613453 Http : // BIL NO
TABUNG AMANAH PINJAMAN PENUNTUT NEGERI PULAU PINANG PEJABAT SETIAUSAHA KERAJAAN NEGERI PULAU PINANG TINGKAT 25, KOMTAR, 10503 PULAU PINANG Tel : 04-6505541 / 6505599 / 6505165 / 6505391 / 6505627 Fax : 04-2613453 Http : //www.penang.gov.my Berikut adalah senarai nama peminjam-peminjam yang telah menyelesaikan keseluruhan pinjaman dan tidak lagi terikat dengan perjanjian pinjaman penuntut Negeri Pulau Pinang Pentadbiran ini mengucapkan terima kasih di atas komitmen tuan/puan di dalam menyelesaikan bayaran balik Pinjaman Penuntut Negeri Pulau Pinang SEHINGGA 31 JANUARI 2020 BIL NO AKAUN PEMINJAM PENJAMIN 1 PENJAMIN 2 TAHUN TAMAT BAYAR 1 371 QUAH LEONG HOOI – 62121707**** NO.14 LORONG ONG LOKE JOOI – 183**** TENG EE OO @ TENG EWE OO – 095**** 4, 6TH 12/07/1995 SUNGAI BATU 3, 11920 BAYAN LEPAS, PULAU PINANG. 6, SOLOK JONES, P PINANG AVENUE, RESERVOIR GARDEN , 11500 P PINANG 2 8 LAU PENG KHUEN – 51062707 KHOR BOON TEIK – 47081207**** CHOW PENG POY – 09110207**** MENINGGAL DUNIA 31/12/1995 62 LRG NANGKA 3, TAMAN DESA DAMAI, BLOK 100-2A MEWAH COURT, JLN TAN SRI TEH EWE 14000 BUKIT MERTAJAM LIM, 11600 PULAU PINANG 3 1111 SOO POOI HUNG – 66121407**** IVY KHOO GUAT KIM – 56**** - 22/07/1996 BLOCK 1 # 1-7-2, PUNCAK NUSA KELANA CONDO JLN 10 TMN GREENVIEW 1, 11600 P PINANG PJU 1A/48, 47200 PETALING JAYA 4 343 ROHANI BINTI KHALIB – 64010307**** NO 9 JLN MAHMUD BIN HJ. AHMAD – 41071305**** 1962, NOORDIN BIN HASHIM – 45120107**** 64 TAMAN 22/07/1997 JEJARUM 2, SEC BS 2 BUKIT TERAS JERNANG, BANGI, SELANGOR. - SUDAH PINDAH DESA JAYA, KEDAH, 08000 SG.PETANI SENTOSA, BUKIT SENTOSA, 48300 RAWANG, SELANGOR 5 8231 KHAIRIL TAHRIRI BIN ABDUL KHALIM – - - 16/03/1999 80022907**** 6 7700 LIM YONG HOOI – A345**** LIM YONG PENG – 74081402**** GOH KIEN SENG – 73112507**** 11/11/1999 104 18-A JALAN TAN SRI TEH, EWE LIM, 104 18-A JLN T.SRI TEH EWE LIM, 11600 PULAU 18-I JLN MUNSHI ABDULLAH, 10460 PULAU PINANG 11600 PULAU PINANG PINANG 7 6605 CHEAH KHING FOOK – 73061107**** NO. -

Sungai Dua Water Treatment Plant Seberang Perai, Malaysia
Sungai Dua Water Treatment Plant Seberang Perai, Malaysia 1. Background Information Sungai Dua Water Treatment Plant (SDWTP) is located at Seberang Perai and occupies about 13 hectares of land. SDWTP is the most important WTP in the Penang as it supplies 80% of the total volume of treated water to Penang. SDWTP was first commissioned in the year 1973 and after series of upgrading in 1994, 1999, 2004, 2011 and 2013, it now has the design capacity of 1,113,792 m3/day . SDWTP usually draws water from Muda River as a primary source and Mengkuang Dam as the secondary source which is the largest dam in the Penang. Currently, the Mengkuang Dam is temporarily decommissioned since February 2014 to facilitate its expansion project. SDWTP is owned and operated by Perbadanan Bekalan Air Pulau Pinang (PBAPP). SDWTP also serves as the control center for PBAPP’s “on-line” supervisory control and data acquisition (SCADA) system to facilitate remote operation. SCADA gathers real-time data from remote location and empowers the operator to remotely control equipment and conditions. The plant operates at 3 shifts per day. The general information of SDWTP is shown in Table 1. Table 1: Overall Information of Sungai Dua Water Treatment Plant Location Seberang Perai, Penang Constructed Year 1973 (1994, 1999, 2004, 2011 & 2013 upgrading) Raw Water Source Muda River (primary) & Mengkuang Dam Maximum Design Capacity (m3/d) 1,113,792 Operating Capacity (m3/d) 1,002,412 Number of employees 120 Topography Tropical Automation Yes Water Quality Exceeds Standards for Drinking Water set by Ministry of Health, Malaysia Supply Areas Seberang Perai, Penang Island Reference Perbadanan Bekalan Air Pulau Pinang (PBAPP) Source: Making Sure Penang’s Taps Keep Flowing. -
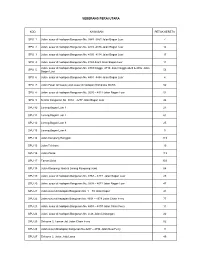
Seberang Perai Utara
SEBERANG PERAI UTARA KOD KAWASAN PETAK KERETA SPU 1 Jalan susur di hadapan Bangunan No. 3843 -3851 Jalan Bagan Luar 7 SPU 2 Jalan susur di hadapan Bangunan No. 4873 -4876 Jalan Bagan Luar 14 SPU 3 Jalan susur di hadapan Bangunan No. 4105 -4114 Jalan Bagan Luar 17 SPU 4 Jalan susur di hadapan Bangunan No. 4144-4223 Jalan Bagan Luar 11 Jalan susur di hadapan Bangunan No. 4703 hingga 4710, 4422 hingga 4425 & 4802 Jalan SPU 5 53 Bagan Luar SPU 6 Jalan susur di hadapan Bangunan No. 4451 -4454 Jalan Bagan Luar 4 SPU 7 Jalan Pasar termasuk jalan susur di hadapan Kompleks MARA 52 SPU 8 Jalan susur di hadapan Bangunan No. 3635 – 4921 Jalan Bagan Luar 51 SPU 9 Sekitar bangunan No. 3914 – 4277 Jalan Bagan Luar 44 SPU 10 Lorong Bagan Luar 1 21 SPU 11 Lorong Bagan Luar 2 61 SPU 12 Lorong Bagan Luar 3 25 SPU 13 Lorong Bagan Luar 4 3 SPU 14 Jalan Kampong Banggali 219 SPU 15 Jalan Telekom 19 SPU 16 Jalan Pantai 113 SPU 17 Taman Selat 303 SPU 18 Jalan Kampong Jawa & Lorong Kampong Jawa 64 SPU 19 Jalan susur di hadapan Bangunan No. 4768 – 4777 Jalan Bagan Luar 25 SPU 20 Jalan susur di hadapan Bangunan No. 3838 – 4072 Jalan Bagan Luar 47 SPU 21 Jalan susur di hadapan Bangunan No. 1 – 79 Jalan Kapal 41 SPU 22 Jalan susur di hadapan Bangunan No. 4654 – 4679 Jalan Chain Ferry 77 SPU 23 Jalan susur di hadapan Bangunan No. 4803 – 4807 Jalan Chain Ferry 11 SPU 24 Jalan susur di hadapan Bangunan No. -

The State of Penang, Malaysia
Please cite this paper as: National Higher Education Research Institute (2010), “The State of Penang, Malaysia: Self-Evaluation Report”, OECD Reviews of Higher Education in Regional and City Development, IMHE, http://www.oecd.org/edu/imhe/regionaldevelopment OECD Reviews of Higher Education in Regional and City Development The State of Penang, Malaysia SELF-EVALUATION REPORT Morshidi SIRAT, Clarene TAN and Thanam SUBRAMANIAM (eds.) Directorate for Education Programme on Institutional Management in Higher Education (IMHE) This report was prepared by the National Higher Education Research Institute (IPPTN), Penang, Malaysia in collaboration with a number of institutions in the State of Penang as an input to the OECD Review of Higher Education in Regional and City Development. It was prepared in response to guidelines provided by the OECD to all participating regions. The guidelines encouraged constructive and critical evaluation of the policies, practices and strategies in HEIs’ regional engagement. The opinions expressed are not necessarily those of the National Higher Education Research Institute, the OECD or its Member countries. Penang, Malaysia Self-Evaluation Report Reviews of Higher Education Institutions in Regional and City Development Date: 16 June 2010 Editors Morshidi Sirat, Clarene Tan & Thanam Subramaniam PREPARED BY Universiti Sains Malaysia, Penang Regional Coordinator Morshidi Sirat Ph.D., National Higher Education Research Institute, Universiti Sains Malaysia Working Group Members Ahmad Imran Kamis, Research Centre and -

MASPEX2015 MALAYSIAN SECONDARY PROPERTY EXHIBITION P E N a N G 13 - 16 August 2015 • Queensbay Mall, Penang
MASPEX2015 MALAYSIAN SECONDARY PROPERTY EXHIBITION P E N A N G 13 - 16 August 2015 • Queensbay Mall, Penang ORGANISING COMMITTEE Mark Saw Organising Chairman MASPEX Penang 2015 Kayte Teh Erick Kho Danny Ooi Kayrens Lee Vice President President Immediate Past President Honorary Secretary Advisor Advisor Advisor Mark Saw - Chairman Danny Ooi - Immediate Past Chairman Kayrens Lee - Honorary Secretary Lena Lim - Honorary Treasurer Jarrone Long - Deputy Head Of Youth Michael Geh - Committee Member Johnny Khoo - Committee Member\ Genny Tse - Committee Member Lena Lim Jarrone Long Michael Geh Johnny Khoo Mary Looi - Committee Member Honorary Treasurer Deputy Head Of Youth Committee Member Committee Member Florence Lim - Committee Member Long Soo Keat - Head Of Youth Celvin Tan - Youth Committee Member Miki Lim - Youth Committee Member Sunny Tse - Youth Committee Member Season Ting - Youth Committee Member Genny Tse Mary Looi Florence Lim Long Soo Keat Committee Member Committee Member Committee Member Head Of Youth Celvin Tan Miki Lim Sunny Tse Season Ting Youth Committee Member Youth Committee Member Youth Committee Member Youth Committee Member MASPEX2015 MALAYSIAN SECONDARY PROPERTY EXHIBITION P E N A N G 13 - 16 August 2015 • Queensbay Mall, Penang PROGRAMME -Thursday, 13th Aug 2015 11.00 am Arrival of Guests 11.30 am Speech by Chairman of MASPEX, Mr Mark Saw 11.40am Speech by MIEA President, Mr Erick Kho 11.55 am Speech by Maybank, Ms Tracy Pan, Head of Mortgage 12 noon Speech by Guest Of Honour, YB Chow Kon Yeow 12.15 pm Official Opening Ceremony 12.30 pm Walk About Exhibition 12.45 pm Press Conference 1.00 pm Lunch PROGRAMME - Saturday, 15th Aug 2015 10.45 am Why Purchase Secondary Properties? Erick Kho, President of MIEA 11.30 am Penang State Government's Affordable Homes and Penang's Transportation Master Plan Y.B. -

WHO TEST PLATES for in VITRO ASSESSMENT of ANTIMALARIAL DRUG SUSCEPTIBILITY Procedures and Conditions for Supply of Test Plates (May 2001)
Communicable Disease Surveillance and Response Vector Control Research Unit School of Biological Sciences (CSR) 11800 Minden, Penang World Health Organization Malaysia 1211 Geneva 27 Tel: 604-6574776 Switzerland Fax: 604-6577200 WHO TEST PLATES FOR IN VITRO ASSESSMENT OF ANTIMALARIAL DRUG SUSCEPTIBILITY Procedures and conditions for supply of test plates (May 2001) The WHO in vitro microtest plates for assessment of parasite susceptibility to antimalarial drugs are produced, from May 2001 by Vector Control Research Unit, Universiti Sains Malaysia, Penang, Malaysia. The test plates will be supplied from Universiti Sains Malaysia (USM), according to the procedures described below. Test kits are no longer produced, as all the items included, except plates, are nowadays easily available from various laboratory suppliers. The low PABA, low folate RPMI medium, which was custom-made for the test kits in the past, is no longer required, as it was needed only for SDX/PYR and PYR tests, for which test plates are no longer produced. Users are recommended to use ordinary RPMI 1640 medium. The only supply item, which is not readily available, is 100 ml heparinized capillary tubes. Investigators are recommended to use two 50 ml tubes (haemotocrit standard) instead. Of course, if a larger number of drugs are to be tested, it is preferable to take venous blood, for example by Vacutainer. 1. Requests Requests should be made to The Coordinator Vector Control Research Unit School of Biological Sciences Universiti Sains Malaysia 11800 Minden, Penang MALAYSIA (Attn: Associate Professor Dr Zairi Jaal) Tel: 604-6574776 Fax: 604-6577200 E-mail: [email protected]. -

Extraordinaryalluring Homes That Inspire Spacious Comfort Home Love and Care Are What a Pleasant Home Is Made Of
ExtraordinaryAlluring homes that inspire spacious comfort Home Love and care are what a pleasant home is made of. Love and care, that’s what Sunway Cassia homes are architected with, and are created towards. Sunway Cassia’s final phase consists of spacious 2-Storey Semi-D Homes that are crafted with these basic tenets in mind. Ardently adorned with a green buffer, these abodes accord you with all the best in life amidst the tropical enclaves of Batu Maung. excellent accessibility high ceilings column-free car porch 2-Storey Semi-D Homes 3-Storey Terrace Homes spacious garden separate wet and dry kitchens Expand your48 exceptional horizon residences that resonate the luxury of space environmental-friendly features amidst 3 acres of greenery Artist’s impression of aerial view Harmony Sunway Cassia makes urban living within a vast verdant landscape a dream come true. Let the little ones learn and grow in the embrace of nature’s enchanting creations and revel in 3 acres of luscious greens. Your home is also built with environmental-friendly features, creating a safe haven for you and your loved ones. Celebrate the bond of family, in homes that are uniquely different. Sanctuary Cydonia Lot size Built-up area 2-Storey Semi-D Homes 35’ x 85’ 3,196 sq ft Your home is where the grass is always greener on your side, and where you can invest your heart and soul into building treasured memories. Ground Floor 1st Floor Artist’s impression of Cydonia 2-Storey Semi-D Homes Sojourn Cydonia+ Lot size Built-up area 2-Storey Semi-D Homes 40’ x 75’ 3,770 sq ft Built tall and spacious, your living space is always illuminated by natural light. -

Choo Kiang Speaker Pulau Pinang, Maktar Timbalan
Penang Transformasi Flat PPR Perang Ramadhan Papan Suoq Jalan MS Sungai Iklan 4 MS10 MS 24 PERCUMA buletin Cekap Akauntabel Telus 1 – 15, JULAI 2013 http:www.facebook.com/buletinmutiara http:www.facebook.com/cmlimguaneng Selamat menunaikan ibadah puasa buat muslimin & muslimat. Choo Kiang Speaker Pulau Pinang, Maktar Timbalan “Saya berharap perjalanan Oleh : ANNAS ZAINUL sidang Dewan berjalan lancar,” ABIDIN & ZAINULFAQAR ujarnya mengalu-alukan YAACOB pembukaan sidang DUN. Katanya, beliau bersama-sama GEORGE TOWN – Penggal Maktar berkongsi cita-cita untuk Pertama Dewan Undangan Negeri menyemarak lagi prinsip demokrasi (DUN) Pulau Pinang Ke-13 secara serta kebebasan bersuara menerusi rasminya dibuka pada pagi Jumaat, DUN Pulau Pinang sepanjang 28 Jun lepas. tempoh lima tahun mendatang ini. Seperti biasa, istiadat pelantikan Dalam perkembangan sama, Speaker DUN dilakukan sebelum Choo Kiang turut mengulas majlis angkat sumpah kesemua Ahli mengenai insiden wartawan akhbar Dewan Undangan Negeri (ADUN) Utusan Malaysia, Mohd. Firdaus dilangsungkan. Ismail, 27, melancarkan protes di Ahli Mesyuarat Dewan luar pagar DUN Pulau Pinang gara- menerima cadangan Ketua Menteri, gara dihalang membuat liputan Y.A.B. Tuan Lim Guan Eng untuk mesyuarat ADUN. melantik ADUN Bukit Tambun, Beliau berkata, larangan itu YANG Di-Pertua Negeri, Tuan Yang Terutama (TYT), Tun Abdul Rahman Abbas menyempurnakan Law Choo Kiang sebagai Speaker adalah ‘era lama’ dan tidak wajar istiadat pemeriksaan perbarisan. DUN Pulau Pinang yang baru. dilakukan pada era beliau menjadi Selain Guan Eng, Timbalan Speaker DUN. mereputasikan lagi peranan DUN Ketua Menteri I, Mohd. Rashid Malah, katanya lagi, beliau tidak Pulau Pinang dalam sistem Hasnon, Prof. Dr. P. Ramasamy akan menghalang mana-mana demokrasi negeri dan Negara. -

Press Statement @ Yb Dato Sri Dr Hj Wan Junaidi Tuanku
PRESS STATEMENT YB DATO SRI DR. HJ WAN JUNAIDI TUANKU JAAFAR MINISTER ENTREPRENEUR DEVELOPMENT AND COOPERATIVES (MEDAC) ON THE PENANG SOUTH RECLAMATION PROJECT ___________________________________________________________________ Putrajaya, 3 Jun 2021 - The Penang South Reclamation Project (Projek Tambakan Laut Selatan Pulau Pinang) -PSR which will take up the size of almost 17 sq km, involves the development of three artificial islands with a land mass of 1,700 hectares in the waters of Permatang Damar Laut near Bayan Lepas in Penang. This proposed development under the Environmental Quality Act 1974 requires an Environmental Impact Assessment Report that needs to be approved by the Department of environment (DOE) which was previously under my charge as the Minister of Natural Resources and Environment (NRE). During this time in 2017 when the matter was brought forth to my attention, I was totally against the project development and even though now the report has been approved on 25 June 2019 by the Department I am still in disagreement with the approval and I wholly support for the project to be cancelled. On this note I have made strong criticisms, substantiated with facts and data, that the project would have serious negative socio-economic and environmental impacts. First and foremost, the EIA report stated that there will be a permanent destruction to the site which will have a significant negative impact on fisheries resources, fishermen and the security of the country’s food supply by affecting the breeding ground for fish, prawns and crabs. The ecosystem of the coast to be reclaimed and the fisheries resources would be permanently destroyed, and the fishing community exposed to grave hardship.