Put Paper Title Here
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Unusual Silicate Mineralization in Fumarolic Sublimates of the Tolbachik Volcano, Kamchatka, Russia – Part 2: Tectosilicates
Eur. J. Mineral., 32, 121–136, 2020 https://doi.org/10.5194/ejm-32-121-2020 © Author(s) 2020. This work is distributed under the Creative Commons Attribution 4.0 License. Unusual silicate mineralization in fumarolic sublimates of the Tolbachik volcano, Kamchatka, Russia – Part 2: Tectosilicates Nadezhda V. Shchipalkina1, Igor V. Pekov1, Natalia N. Koshlyakova1, Sergey N. Britvin2,3, Natalia V. Zubkova1, Dmitry A. Varlamov4, and Eugeny G. Sidorov5 1Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia 2Department of Crystallography, St Petersburg State University, University Embankment 7/9, 199034 St. Petersburg, Russia 3Kola Science Center of Russian Academy of Sciences, Fersman Str. 14, 184200 Apatity, Russia 4Institute of Experimental Mineralogy, Russian Academy of Sciences, Akademika Osypyana ul., 4, 142432 Chernogolovka, Russia 5Institute of Volcanology and Seismology, Far Eastern Branch of Russian Academy of Sciences, Piip Boulevard 9, 683006 Petropavlovsk-Kamchatsky, Russia Correspondence: Nadezhda V. Shchipalkina ([email protected]) Received: 19 June 2019 – Accepted: 1 November 2019 – Published: 29 January 2020 Abstract. This second of two companion articles devoted to silicate mineralization in fumaroles of the Tol- bachik volcano (Kamchatka, Russia) reports data on chemistry, crystal chemistry and occurrence of tectosil- icates: sanidine, anorthoclase, ferrisanidine, albite, anorthite, barium feldspar, leucite, nepheline, kalsilite, so- dalite and hauyne. Chemical and genetic features of fumarolic silicates are also summarized and discussed. These minerals are typically enriched with “ore” elements (As, Cu, Zn, Sn, Mo, W). Significant admixture of 5C As (up to 36 wt % As2O5 in sanidine) substituting Si is the most characteristic. Hauyne contains up to 4.2 wt % MoO3 and up to 1.7 wt % WO3. -

Lapis Lazuli
LAPIS LAZULI Among the world’s many gemstones, lapis lazuli, or “lapis” as it is familiarly known, has a distinction. Many scholars and archaeologists believe that it was the first gemstone ever mined in quantity. The oldest known lapis deposits, located in Afghanistan, have been systematically mined since about 4000 B.C. Few gemstones display color as rich as the deep, royal blue of fine lapis. Adding to its visual appeal, lapis often exhibits glittering, golden flecks of pyrite. When cut and polished into cabochons and set in gold, lapis gemstones have a distinctive beauty all their own. Understanding lapis lazuli, which is a rock, requires a basic understanding of lazurite, which is a mineral. Lazurite is the primary component of lapis lazuli and is also the cause of its blue color. Lazurite, or basic sodium calcium aluminum sulfate chlorosilicate (Na,Ca)8Si6Al6O24[(SO4),S,Cl,(OH)]2, is a tectosilicate or framework silicate that is made up of 13.5 percent sodium, 7.8 percent calcium, 15.8 percent aluminum, 16.3 percent silicon, 6.0 percent sulfur, 38.1 percent oxygen, and small percentages of chlorine and hydrogen. The color of pure lazurite is a deep azure. It crystallizes in the isometric (cubic) system, usually as dodecahedral and occasionally as cubic crystals, but most often occurs in massive form. Lazurite is usually opaque, has a dull-to-greasy luster, poor cleavage in six directions, a pale-blue streak, a Mohs hardness of 5.0-5.5, and a specific gravity of 2.4-2.5. Lazurite forms from high-grade, contact metamorphism of silica-poor marine limestone that contains available sulfur and chlorine. -

Recovery of Desilication Product in Alumina Industry
Recovery of Desilication Product in Alumina Industry Hasitha Indrajith de Silva B.Sc. Engineering (Honours) A thesis submitted for the degree of Doctor of Philosophy at The University of Queensland in 2013 School of Chemical Engineering Abstract Bayer process is used for the production of alumina from bauxite which contains siliceous minerals known as reactive silica. Reactive silica is also digested during the Bayer process, forming desilication product (DSP) which traps significant amount of sodium from the caustic soda used. DSP containing red mud is discarded to the bauxite residue storage areas causing alarming economic and environmental concerns to the alumina industry. Separation of DSP from red mud is a critical step to subsequent sodium recovery. Therefore, methods of separating DSP from red mud originated in a Western Australian alumina refinery were investigated in the present study. Red mud classified into five size classes was characterised using particle size measurement, material density, X-ray diffraction, X-ray photon spectroscopy, electron microscopy fitted with energy dispersive spectroscopy. Size separation alone resulted in an increase of DSP from ~7% to 12-14% in finer size classes of red mud. Since red mud contains more than 50% iron oxides, dissolution of these oxides would invariably result in an increase of DSP, therefore, several dissolution mechanisms of these oxides were investigated. Sodium Citrate-Bicarbonate-Dithionite (CBD) method resulted in an increase of DSP content from ~12 to 22%, while a comparative study was also carried out using deferroxiamine B (DFO-B) as the complexing agent. The use of ultrasonically assisted-CBD resulted in a significant improvement of iron dissolution from red mud. -

Phase Relations of the Hydroxyl Felspathoids in the System Naalsi0 -Naoh-H 0 4 2
THE HYDROXYL FELSPATHOIDS PHASE RELATIONS OF THE HYDROXYL FELSPATHOIDS IN THE SYSTE14 NaAlSiO4-NaOH-H 0 2 By PETER ASCROFT MACKENZIE ANDERSON, B.Se., M.Se. A Thesis Submitted to the Faculty of Graduate Studies in Partial Fulfilment of the Requirements for the Degree Doetor of Philosophy McMaster University May 1968 DOCTOR OF PHILOSOPHY (1968) McMASTER UNIVERSITY (Geology) Hamilton, Ontario TITLE: Phase Relations of the Hydroxyl Felspathoids in the System NaAlSi0 -NaOH-H 0 4 2 AUTHOR: Peter A.M. Anderson, B.Sc. (London University) M.Sc. (McMaster University) SUPERVISOR: Dr. B.J. Burley NUMBER OF PAGES: ix, 78 SCOPE AND CONTENTS: A stUdy was made of the phase relationships in the system NaAlSi0 -NaOH-H 0, at 15,000 p.s.i. and at 450°C, 520°C, 4 2 600°C and 700°C. The following phases were found and characterised: hydroxyl varieties of nosean, sodalite and cancrinite, with formulae approximating to 3NaA1Si04.NaOH.xH20, l6:.xbl.5, sodic nepheline and phase X, apparently a previously unreported phase, with composition approaching Na Al Si 0 • Approximate phase diagrams were deduced 4 2 2 9 for the four temperatures. i1 ACKNOWLEOOEMENTS The writer is very deeply indebted to Dr. B.J. Burley, without whose continued guidance and support, this investigation would have been quite impossible. Drs. C. Calvo and H.P. Schwarcz have also been generous with time and advice, throughout the project. The assist- ance of others, too numerous to mention individually, has been readily given and gratefully accepted; these include most of the members of the Department of Geology. -

A Chemical, Thermogravimetric and X-Ray Study of Cancrinite
A CHEMICAL, THERMOGRAVIMETRIC AND X-RAY STUDY OF CANCRINITE by 1 SHU-MEEI CHEN, B • Sc. A The sis Submitted to the School of Graduate Studies in Partial Fulfilment of the Requirements for the Degree Master of Science McMaster University September, 1970 STUDIES ON CANCRINITE MASTER OF SCIENCE (Ge ology) McMASTER UNIVERSITY Hamilton, Ontario. TITLE: A Chemical, Thermogravimetric and X-Ray Study of C ancrinite. AUTHOR: Shu-Meei Chen, B.Sc. (National Taiwan University) SUPER VISOR: Professor B. J. Burley NUMBER OF PAGES: 65, viii. SCOPE AND CONTENTS: Four specimens of natural cancrinite from Ontario we r e studied. Single crystal photog raphs were taken and non-Bragg reflections were observed. Changes in these reflections in samples heated to 410°, 600°, and 745°C "\Vere investigated. Phase changes in these heated cancrinites we re also studied. Suggestions have been n1ade as to the origin of the non-Bragg reflections. ii ACKNOWLEDGEMENTS The writer wishes to express her sincere appreciation to Professor B. J . Burley, her supervisor, for many discussions and c orrections of the manuscript. Thanks are also extended to Dr. H. D. Grundy for his help with taking precession photographs, to J. Muysson, who did the chemical analyses and to F. Tebay, who took care of the x -ray equipment. Financial support was provided by the Department of Geology, McMaster University, the National Re search Council and the Geological Survey of Canada. iii TABLE OF CONTENTS PAG:E CHAPTER 1 INTRODUCTION 1 CHAPTER 2 STUDIES ON PRECESSION PHOTOGRAPHS OF CANCRINITE -
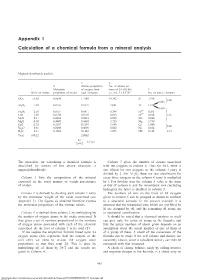
Appendix 1 Calculation of a Chemical Formula from a Mineral Analysis
Appendix 1 Calculation of a chemical formula from a mineral analysis Appendix 1 Magnesiohornblende analysis 3 4 2 Atomic proportion No. of anions on 1 Molecular of oxygen from basis of 24 (O,OH) 5 Wt.% of oxides proportion of oxides each molecule i.e. col. 368.3735 No. of ions in formula SiO 51.63 0.8594 1.7188 14.392 Si 7.196 2 8.00 0.804 } Al2O3 7.39 0.0725 0.2175 1.821 Al 1.214 0.410 3+ Fe2O3 2.50 0.0157 0.0471 0.394 Fe 0.263 FeO 5.30 0.0738 0.0738 0.618 Fe2+ 0.618 5.07 MnO 0.17 0.0024 0.0024 0.020 Mn 0.020 } MgO 18.09 0.4489 0.4489 3.759 Mg 3.759 CaO 12.32 0.2197 0.2197 1.840 Ca 1.840 2.00 Na2O 0.61 0.0098 0.0098 0.082 Na 0.164 } H2O+ 2.31 0.1282 0.1282 1.073 OH 2.146 2.15 Total 100.32 2.8662 24 = 8.3735 2.8662 The procedure for calculating a chemical formula is Column 5 gives the number of cations associated described by means of the above example, a with the oxygens in column 4. Thus for SiO2 there is magnesiohornblende. one silicon for two oxygens so the column 4 entry is divided by 2. For A12O3 there are two aluminiums for Column 1 lists the composition of the mineral every three oxygens so the column 4 entry is multiplied expressed in the usual manner as weight percentages by ~˜. -

Hettmann Et Al (2012): the Sulfur Speciation in S-Bearing Minerals
American Mineralogist, Volume 97, pages 1653–1661, 2012 The sulfur speciation in S-bearing minerals: New constraints by a combination of electron microprobe analysis and DFT calculations with special reference to sodalite-group minerals KAI HETTMANN,* THOMAS WENZEL, MICHAEL MARKS, AND GREGOR MARKL Fachbereich Geowissenschaften, Eberhard-Karls Universität Tübingen, Wilhelmstrasse 56, 72072 Tubigen, Germany ABSTRACT In this work, we present an improved method for the semi-quantitative determination of sulfur species in S-bearing minerals by electron microprobe analysis. For calibration, we analyzed several sulfate and sulfide standard minerals such as baryte, celestine, chalcopyrite, and pyrite, and correlated the results with theoretical calculations retrieved from density functional theory (DFT). We applied this method to natural sodalite-group minerals from various localities. In addition, we applied the more common Raman spectroscopy to some samples and show that this method cannot be applied to sodalite-group minerals to determine their sulfur speciation. We show that even though sodalite-group minerals have a complex crystal structure and are sensitive to the electron beam, electron micro- probe analysis is a reliable tool for the analysis of their sulfur speciation. The natural sodalite-group minerals show systematic variations in sulfur speciation. These variations can be correlated with the independently determined oxidation state of the parental magmas thus making S-bearing sodalite- group minerals good redox proxies, although -

Spectroscopic and Crystal-Chemical Features of Sodalite-Group Minerals from Gem Lazurite Deposits
minerals Article Spectroscopic and Crystal-Chemical Features of Sodalite-Group Minerals from Gem Lazurite Deposits Nikita V. Chukanov 1,2,*, Anatoly N. Sapozhnikov 3, Roman Yu. Shendrik 3 , Marina F. Vigasina 2 and Ralf Steudel 4 1 Institute of Problems of Chemical Physics, Russian Academy of Sciences, Chernogolovka, 142432 Moscow, Russia 2 Faculty of Geology, Moscow State University, Vorobievy Gory, 119991 Moscow, Russia; [email protected] 3 Vinogradov Institute of Geochemistry, Siberian Branch of Russian Academy of Sciences, 1a Favorskii St., 664033 Irkutsk, Russia; [email protected] (A.N.S.); [email protected] (R.Y.S.) 4 Institute of Chemistry, Technical University Berlin, D-10623 Berlin, Germany; [email protected] * Correspondence: [email protected]; Tel.: +7-4965221556 Received: 5 November 2020; Accepted: 20 November 2020; Published: 23 November 2020 Abstract: Five samples of differently colored sodalite-group minerals from gem lazurite deposits were studied by means of electron microprobe and wet chemical analyses, infrared, Raman, electron spin resonance (ESR) and UV-Visible spectroscopy, and X-ray diffraction. Various extra-framework 2 2 components (SO4 −,S − and Cl− anions, S3•−,S2•− and SO3•− radical anions, H2O, CO2, COS, cis- as well as trans- or gauche-S4 neutral molecules have been identified. It is shown that S3•− and S4 are the main blue and purple chromophores, respectively, whereas the S2•− yellow chromophore and SO3•− blue chromophore play a subordinate role. X-ray diffraction patterns of all samples of sodalite-group minerals from lazurite deposits studied in this work contain superstructure reflections which indicate different kinds of incommensurate modulation of the structures. -

Lapis Lazuli
www.saltworkconsultants.com Salty MattersJohn Warren - Friday Nov 13, 2015 Lapis Lazuli: one of the many metamorphosed evaporite pre- cious stones and gems Introduction solution/transformation of meta-evaporites facilitates the for- Precious stones and gems are rare by definition; hence need ex- mation of open fluid space (sometimes pressurized) in veins ceptional geologic conditions to give rise to gem-quality mate- and fractures so favoring sites that then allow the free growth rials. A nexus across most natural gem-forming environments is of precious stones and euhedral ruby, tourmaline and emerald the requirement for hydrous typically saline to hypersaline solu- gemstones. tions, apt to precipitate euhedral crystals in a void or a pressure I am not saying all precious stones and gems are associated with shadow, from fluids that contain elevated and unusual levels of evaporites, many natural gemstone settings are not, but the lapis particular constituents, including chromophores; hence pegma- lazuli of Afghanistan, the melon-sized perfect rubies of Myan- tites, volcanics and meta-evaporites are commonplace hosts for mar, the prolific emerald fields in Columbia, and the tsavorite natural gemstones. Fluids promoting the growth of gem-quali- deposits of east Africa likely are (Garnier et al., 2008; Giuliani ty crystals typically include the availability of uncommon major et al 2005; Feneyrol et al., 2013). In this article I will focus on constituents, along with the presence of adequate chromophores lapis lazuli, for a discussion of other semi-precious stones and , as well limited concentrations of undesirable elements. Another gems that are meta-evaporites see Chapter 14 in Warren (2016). -

Kinetic and Thermodynamic Studies on Adsorption Behaviour of Rhodamine B Dye on Nosean Synthesised from Coal Fly Ash
© 2018 JETIR December 2018, Volume 5, Issue 12 www.jetir.org (ISSN-2349-5162) KINETIC AND THERMODYNAMIC STUDIES ON ADSORPTION BEHAVIOUR OF RHODAMINE B DYE ON NOSEAN SYNTHESISED FROM COAL FLY ASH. 1Sanjay R. Kankrej, 2Mayuri S. Kulkarni, 3Rajendra P. Patil, 4Ashok V. Borhade 1 Associate professor in Chemistry, 2Assistant professor in Chemistry, 3 Associate professor in Chemistry, 4Associate professor in Chemistry 1Department of Chemistry, 1Bhonsala Military College, Nashik - 422005, India. ABSTRACT Release of colouring agents like Rhodamine B dye in water bodies is an important environmental problem as it is carcinogenic and non-bio-degradable. On the other hand plethora of coal fly ash (CFA) produced in thermal power stations is another threat to the environment. Considering these problems; present study deals with recyclability and modification of waste 0 coal fly ash into alumino-silicate Nosean [Na8Al6Si6O24(SO4).H2O] by fusing CFA with alkali at 550 C followed by hydrothermal treatment. The synthesised nosean was fully characterized by FT-IR, XRD, SEM and BET surface area and it was further checked for potential application for removal of the dye Rhodamine B from its aqueous solutions using batch method. The obtained equilibrium data were fitted by the Langmuir, Freundlich, and Temkin and Dubinin-Radushkevich isotherm models. The well-known thermodynamic parameters such as change in Gibb’s free energy (ΔG), entropy change (ΔS) and enthalpy change (ΔH) were evaluated first time for nosean to check the possibility of adsorption process. Further the kinetic parameters such as rate constant and order of adsorption process is also estimated. Index Terms- Fly ash, hydrothermal, Nosean, adsorption, Rhodamine B. -

LAPIS LAZULI from the COQUIMBO REGION, CHILE by Robert R
LAPIS LAZULI FROM THE COQUIMBO REGION, CHILE By Robert R. Coenraads and Claudio Canut de Bon Lapis lazuli has been mined from the apis lazuli is a rock composed of lazurite—the Coquimbo Region of Chile since 1905. Some source of the blue color—with variable amounts of this material approaches the quality of fine of other minerals depending on its origin and, typ- lapis lazuli from Afghanistan. The Chilean ically, small particles of pyrite. Prized for its attractive blue material is composed of blue lazurite, together L color, lapis lazuli was used in jewelry by some of the world’s with wollastonite, calcite, haüyne, diopside, most ancient civilizations. The stone is mined at relatively pyrite, and minor quantities of other minerals. few locations, some of which have been worked since the The deposit is located in the Andes Mountains at an elevation of 3,500 m; it is hosted by a fifth millennium BC (von Rosen, 1990). contact-metamorphosed limestone that was Considering the extensive history and romance attached later metasomatized to introduce sulfur, a to this ornamental gem material, the Chilean deposit is a necessary component for the formation of relative newcomer. The first reference to Chilean lapis lazurite. Two companies are currently mining lazuli dates back to the 19th century (Field, 1850a and b). the deposit, Las Flores de Los Andes S.A. and Another early mention was made by Ignacio Domeyko Compañía Minera LapisChile S.A., and today (1860), a Polish immigrant and mining engineer who they produce about 150 tonnes of material became the first director of the School of Mines at La annually. -

10420 Sodalite
10420 Sodalite Very light white-blue, semi precious stone Chemical Formula: Na 4Al 3(SiO 4)3Cl, Sodium Aluminum Silicate Chloride Molecular Weight: 969.21 gm Sodalite is a rare, rich royal blue mineral widely enjoyed as an ornamental stone. Although massive sodalite samples are opaque, crystals are usually transparent to translucent. Sodalite is a member of the sodalite group and together with hauyne, nosean and lazurite is a common constituent of lapis lazuli. Discovered in 1806 in Greenland, sodalite did not become important as an ornamental stone until 1891 when vast deposits of fine material were discovered in Ontario, Canada. It has since been named Princess Blue after Princess Patricia who, upon visiting Ontario some time after its discovery, chose sodalite as interior decoration for Marlborough House in England. Properties A light, relatively hard yet fragile mineral, sodalite is named after its sodium content; in mineralogy it may be classed as a feldspathoid. Well known for its blue colour, sodalite may also be grey, yellow, green, or pink and is often mottled with white veins or patches. The more uniformly blue material is used in jewellery, where it is fashioned into cabochons and beads. Lesser material is more often seen as facing or inlay in various applications. Although very similar to lazurite and lapis lazuli, sodalite is never quite comparable, being a royal blue rather than ultramarine. Sodalite also rarely contains pyrite, a common inclusion in lapis. It is further distinguished from similar minerals by its white (rather than blue) streak. Sodalite's six directions of poor cleavage may be seen as incipient cracks running through the stone.