Functional Diversity Mediates Macroecological Variation in Plant–Hummingbird Interaction Networks
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Functional Diversity Mediates Macroecological Variation in Plant–Hummingbird Interaction Networks
Received: 21 June 2017 | Revised: 13 May 2018 | Accepted: 22 May 2018 DOI: 10.1111/geb.12776 RESEARCH PAPER Functional diversity mediates macroecological variation in plant–hummingbird interaction networks Pietro Kiyoshi Maruyama1,2,3 | Jesper Sonne2 | Jeferson Vizentin‐Bugoni4,5 | Ana M. Martín González2 | Thais B. Zanata2,6 | Stefan Abrahamczyk7 | Ruben Alarcón8 | Andréa C. Araujo9 | Francielle P. Araújo10 | Andrea C. Baquero2 | Edgar Chávez‐González11 | Aline G. Coelho12 | Peter A. Cotton13 | D. Matthias Dehling14,15 | Erich Fischer9 | Glauco Kohler16 | Carlos Lara17 | Flor Maria G. Las‐Casas18 | Adriana O. Machado3 | Caio G. Machado12 | María A. Maglianesi14,19 | Tiago S. Malucelli6 | Oscar Humberto Marín‐Gómez20 | Paulo E. Oliveira3 | Juan Francisco Ornelas21 | Raul Ortiz‐Pulido22 | Mónica B. Ramírez‐Burbano23 | Márcia A. Rocca24 | Licléia C. Rodrigues25 | Liliana Rosero‐Lasprilla26 | Ana M. Rui27 | Brody Sandel28 | Jens‐Christian Svenning29,30 | Boris A. Tinoco31 | Isabela G. Varassin6 | Stella Watts32 | Carsten Rahbek2,33 | Marlies Sazima1 | Matthias Schleuning14 | Bo Dalsgaard2 1Departamento de Biologia Vegetal, Instituto de Biologia, Universidade Estadual de Campinas (Unicamp), Campinas, Brasil 2Center for Macroecology, Evolution and Climate, Natural History Museum of Denmark, University of Copenhagen, Copenhagen Ø, Denmark 3Instituto de Biologia, Universidade Federal de Uberlândia, Uberlândia, Brasil 4Programa de Pós‐Graduação em Ecologia, Universidade Estadual de Campinas (Unicamp), Campinas, Brasil 5Natural Resources and Environmental -

Cortes-Diago Et Al MS-636.Fm
ORNITOLOGIA NEOTROPICAL ________________________________________________________________________ Volume 18 2007 No. 2 ________________________________________________________________________ ORNITOLOGIA NEOTROPICAL 18: 161–170, 2007 © The Neotropical Ornithological Society A NEW SPECIES OF ERIOCNEMIS (TROCHILIDAE) FROM SOUTHWEST COLOMBIA Alexander Cortés-Diago1, Luis Alfonso Ortega2, Luis Mazariegos-Hurtado1, & André-A. Weller3 1The Hummingbird Conservancy, Calle 17 A No. 121-11, Cali, Colombia. E-mail: [email protected] 2Fundación Ecohabitat, Calle 64 AN No. 10-71, Popayán, Colombia. 3Zoological Research Museum A. Koenig, Biology and Phylogeny of Tropical Birds, Adenauerallee 160, 53113 Bonn, Germany. Resumen. – Una nueva especie de Eriocnemis (Trochilidae) del suroeste de Colombia. – Una nueva especie de colibrí, el Zamarrito del Pinche (Eriocnemis isabellae, sp. nov.), es descrita de la Serranía del Pinche, un macizo aislado e inexplorado localizado en el Departamento del Cauca en el suroeste de Colombia (02°16’04.18”N, 77°21’26.41”W, 2800 m s.n.m.). Esta especie representa un nuevo miembro distincto del género Eriocnemis y habita los bosques templados y nublados de la Serranía. Aunque se puede identificar fácilmente como un miembro del género Eriocnemis por sus zamarros blancos, el azul violeta en las infracaudales de la cola y la cola azul negra bifurcada, se diferencia ampliamente de la mayoría de las especies de su género en tener la cara, corona y nuca de color negro con visos amarillosos verde oliva. Además, tiene una gorguera bicolor iridiscente distincta, azul violeta y verde. Este nuevo taxón comparte algunas características con otros de su género (i.e., E. vestitus, E. nigrivestis) y esta ecológicamente asociado a bosques enanos ocupando un pequeño rango en pendientes pronunciadas a lo largo de filos montañosos. -

Rediscovery of the Colourful Puffleg Eriocnemis Mirabilis
Rediscovery of the Colourful Puffleg Eriocnemis mirabilis Luis A. Mazariegos H. and Paul G. W. Salaman Cotinga 11 (1999): 34–38 Descubierto en 1967, el enigmático Colibrí de Zamarros Eriocnemis mirabilis es conocido de 4 ejemplares, colectados en la ladera de un cerro de la vertiente pacífica de la Cordillera Occidental en Colombia. Información erronea sobre la localidad, y una busqueda intensiva redescubrieron la especie finalmente, confirmada por evidencia fotográfica en noviembre de 1997. Visitas repetidas al sitio y áreas adyacentes revelaron el microendemismo de la especie en el Parque Nacional Natural Munchique posiblemente relacionado a requerimientos ecológicos específicos en un angosto rango altitudinal de bosque bajo humedo montano. E. mirabilis enfrenta una situación delicada de conservación, directamente amenazado por Colonos ilegales en su localidad dentro del Parque Nacional. Un proyecto se está realizando para proveer de una alternativa económica a la explotación de madera y además se require un estudio ecológico urgente y detallado sobre la especie. Este artículo es dedicado a la memoria de un gran ornitólogo Colombiano, Alvaro Negret. Introduction Parque Nacional Natural (PNN) Munchique, on the Pacific slope of the Cordillera Occidental of Colombia in the Department of Cauca, encompasses 44,000 ha of wet foothill to montane cloud forest at 500–3,012 m. The area was declared a Sanctuario de Fauna y Flora in 1967 following the discovery of a new hummingbird species—Colorful Puffleg Eriocnemis mirabilis3,6—and constitutionally became a National Park (PNN) in 1977. During the past three decades, many attempts have been made by birders, ornithologists and park staff alike to relocate E. -

Appendix 1 – Original Contract Specification
MAPISCo Final report: Appendix 1 – Original contract specification Appendices Appendix 1 – Original contract specification Methodology for Assessment of priorities for international species conservation (MAPISCo) Competition Details and Project Specification Competition Code: WC1017 Date for return of tenders: 2nd August 2011 Address for tender submission: Competition Code: WC1017 (the Competition Code must be shown Defra on the envelope and the tender Natural Environment Science Team submitted in line with the instructions in Zone 1/14 the attached guidance, otherwise your Temple Quay House tender may not be accepted) 2 The Square Temple Quay Bristol BS1 6EB Number of electronic & hard copies 1 copy on CD-ROM or 3½” disk, plus required: [2] hard copies Contact for information relating to this Name: Dominic Whitmee project specification: Tel no: 0117 372 3597 e-mail: [email protected] Proposed ownership of Intellectual Defra Property (contractor or Defra): Proposed start-date (if known): September 2011 Proposed end-date (if known): September 2012 Project Specification BACKGROUND The UK government is committed to the conservation and sustainable use of biodiversity. A wide range of domestic policies and national and EU legislation are in place which contribute to this objective and assist in the conservation of threatened species, habitats and ecosystems, either directly such as measures that control the keeping or sale of specified species, or indirectly such as controls on the release of pollutants into water courses or the atmosphere. Internationally the UK is a significant player in a number of multilateral environment agreements and related initiatives which aim to support the conservation and sustainable use of species, habitats and ecosystems. -

Global Patterns of Interaction Specialization in Bird–Flower Networks
DOI: 10.1111/jbi.13045 ORIGINAL ARTICLE Global patterns of interaction specialization in bird–flower networks Thais B. Zanata1,2,3,* | Bo Dalsgaard3 | Fernando C. Passos4 | Peter A. Cotton5 | James J. Roper1,6 | Pietro K. Maruyama7 | Erich Fischer8 | Matthias Schleuning9 | Ana M. Martın Gonzalez 3,10 | Jeferson Vizentin-Bugoni11,12 | Donald C. Franklin13 | Stefan Abrahamczyk14,15 | Ruben Alarcon 16 | Andrea C. Araujo17 | Francielle P. Araujo 18 | Severino M. de. Azevedo-Junior19 | Andrea C. Baquero3 | Katrin Bohning-Gaese€ 9,20 | Daniel W. Carstensen3 | Henrique Chupil21 | Aline G. Coelho22 | Rogerio R. Faria23 | David Horak 24 | Tanja T. Ingversen25 | St ep an Janecek24,26 | Glauco Kohler1,27 | Carlos Lara28 | Flor M. G. Las-Casas29 | Ariadna V. Lopes30 | Adriana O. Machado31 | Caio G. Machado22 | Isabel C. Machado30 | Marıa A. Maglianesi9,32 | Tiago S. Malucelli1,2 | Jayasilan Mohd-Azlan13,33 | Alan C. Moura22 | Genilda M. Oliveira34 | Paulo E. Oliveira31 | Juan Francisco Ornelas35 | Jan Riegert36 | Licleia C. Rodrigues37 | Liliana Rosero-Lasprilla38 | Ana M. Rui39 | Marlies Sazima7 | Baptiste Schmid40 | Ondrej Sedlacek24 | Allan Timmermann41 | Maximilian G. R. Vollstadt€ 9,20 | Zhiheng Wang42 | Stella Watts43 | Carsten Rahbek3,44 | Isabela G. Varassin2 1Programa de Pos-Graduac ßao~ em Ecologia e Conservacß~ao, Universidade Federal do Parana, Curitiba-PR, Brazil 2Laboratorio de Ecologia Vegetal, Departamento de Bot^anica, Universidade Federal do Parana, Curitiba, Brazil 3Center for Macroecology, Evolution and Climate, Natural History -
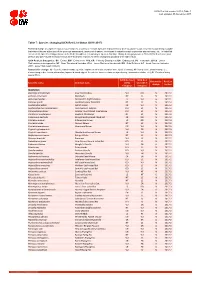
Table 7 Last Updated: 05 December 2017
IUCN Red List version 2017-3: Table 7 Last Updated: 05 December 2017 Table 7: Species changing IUCN Red List Status (2016-2017) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2016 (IUCN Red List version 2016.3) and 2017 (IUCN Red List version 2017-3) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2016) List (2017) change version Category Category MAMMALS Allactaga tetradactyla Four-toed Jerboa VU DD N 2017-2 Antilope cervicapra Blackbuck NT LC N 2017-2 -

Bird Reserve Network
The Latin American BIRD RESERVE NETWORK S TOPPING EXTINCTION IN ITS TRACKS American Bird Conservancy (ABC) was founded in 1994, and is the only not-for-profit organization that works solely to conserve native wild birds and their habitats throughout the Americas. ABC acts to safeguard the rarest birds, conserve habitat for declining species, eliminate threats to bird populations, develop innovative solutions to conservation problems, and to build capacity in the bird conservation movement. ABC tracks all bird issues, responds rapidly to the highest conservation priorities, and works constructively with stakeholders to seek win-win solutions that will be effective in the long-term. ABC takes a flexible approach to problem solving, and its programs range from land purchase and restoration to advocacy and education. To help develop the reserve network, ABC identifies priority sites, provides technical support and training, raises funds, monitors and evaluates the progress of conservation action at each site, and provides project management assistance to partners. ABC’s goal is to expand the reserve network to conserve all endangered bird species in the Americas, for each reserve to attain ABC’s Gold Standard of Operational Excellence, and to achieve long-term financial sustainability. Jocotoco Antpitta (AZE, EN, 1): Mark Harper PHOTOGRAPHIC CAPTIONS After each bird species’ name we state whether it is an Alliance for Zero Extinction (AZE) species (see page www.abcbirds.org 107); we include its IUCN status (CR: critically endangered; EN: endangered; VU: vulnerable; NT: near- threatened; LC: least concern); if it is a country endemic or probable endemic (prob.) the country; and the number of ABC-supported reserves where the species occurs. -

AZE.Qrk 11/14/05 5:14 PM Page 1
AZE.Qrk 11/14/05 5:14 PM Page 1 Mexico/Isla Socorro (MX34) Photo by Mike Parr ALLIANCE FOR Volcano rabbit Romerolagus diazi Azores bullfinch Pyrrhula murina Mexico/Sur del Valle de México (MX55) Portugal/Azores, east of São Miguel (PO1) Photo by Alejandro Velázquez Zero Extinction Photo by Leo J.R. Boon/Cursorius Pinpointing and Preventing Imminent Extinctions • www.zeroextinction.org Whooping crane Grus americana Asia Minor spiny mouse Costa Rica/Osa US/Aransas National Wildlife Refuge (US2), Acomys cilicicus Peninsula (CR2) Canada/Wood Buffalo National Park (CA2) Turkey/Silifke (TU2) Photo by Mike Parr Photo by USFWS he Alliance for Zero Extinction (AZE), a joint initia- Photo by Ahmet Karatas tive of 52 biodiversity conservation organizations, T aims to prevent extinctions by identifying and safe- guarding key sites where species are in imminent danger of disappearing. The goal of the Alliance is to create a front Flat-backed spider tortoise Pyxis planicauda Madagascar/Menabe Forest (MA13) Dusky gopher frog Rana sevosa line of defense against extinction by eliminating threats and Photo by John Behler, Bronx Zoo/WCS US/Glen's Pond in Mont Nimba viviparous toad Okinawa rail US10 Worthen's sparrow Spizella wortheni De Soto National Forest (US5) Nimbaphrynoides occidentalis Gallirallus okinawae Photo by Suzanne L. Collins/The Center Guinea/Mont Nimba (GU1) Mexico/Saltillo savanna (MX47) Torrey pine Pinus torreyana restoring habitat to allow species populations to rebound. Japan/Yambaru (JA8) Photo by Ray Bieber for North American Herpetology -

Songbird Remix Hummingbirds of South America
Avian Models for 3D Applications by Ken Gilliland 1 Songbird ReMix Hummingbirds of the Americas Volume II Contents Manual Introduction 3 Overview and Use 3 Conforming Crest Quick Reference 4 Creating a Songbird ReMix Bird 5 Using Conforming Crests with Poser 6 Using Conforming Crests with DAZ Studio 8 Field Guide – Hummingbirds of South America List of Species 9 General Notes about Hummingbirds 10 Lancebills, Barbthroats & Hermits Reddish Hermit 12 Sicklebills, Sabrewings & Jacobins Buff-tailed Sicklebill 13 Violet Sabrewing 15 Swallow-tailed Hummingbird 16 Topazes & Mangoes Ruby Topaz 18 Coquettes & Thorntails Frilled Coquette 19 Rufous-crested Coquette 20 Emeralds, Sapphires, Brilliants, Hillstars & Incas Sword-billed Hummingbird 21 Black Inca 22 Starfrontlets, Sunangels & Firecrowns Royal Sunangel 24 Juan Fernández Firecrown 26 Pufflegs Colorful Puffleg 28 Trainbearers, Thornbills, Cornets, Starthroats & Woodstars Marvelous Spatuletail 30 Bearded Helmetcrest 32 Resources, Credits and Thanks 33 Wing Motion Blurs Tutorials 34 Scaling Alters Bump/Displacement and Rendering Tips for 3D Applications 38 Copyrighted 2011-2013 by Ken Gilliland songbirdremix.com Opinions expressed on this booklet are solely that of the author, Ken Gilliland, and may or may not reflect the opinions of the publisher. 2 Songbird ReMix Hummingbirds of the Americas Volume II Introduction “Hummingbirds of the Americas” adds the smallest of songbirds to the Songbird ReMix series. While endemic to the Americas, they have been adopted worldwide as one of the most popular and beloved songbirds. Hummingbirds have been a staple in both ancient and modern cultures as a sign of vigor, energy, and skill. This collection focuses on hummingbirds found throughout South America, from the feeders of the Rio de Janeiro suburbs to the tops of the Andes. -

Annual Report 2005
Annual Report 2005 American Bird Conservancy—the future of bird conservation AMERICAN BIRD CONSERVANCY ABC is leading the way in addressing the top priority threats From the Chairman to birds, and is developing a comprehensive program to halt extinctions and restore populations. For example, there write this in my last year as Chair of ABC’s are 88 sites in the Americas where a bird species is down to Board before I rotate off under our term its last stronghold. Many remain unprotected. With your limit policy that ensures a healthy turnover help, ABC will try to protect as many as half these sites in Iof Directors. During my nine years on the the next five years. Keystone wildlife legislation, such as the Board, I have seen ABC grow dramatically in prominence, Endangered Species Act, is under relentless attack and can capability, and achievement. Who could have guessed only be protected if organizations such as ABC show creative that a small bird group with an initial total budget of a leadership, as demonstrated by our recent report showing few hundred thousand dollars could expand so rapidly the positive impact of the Act on endangered birds. “Bird that it could successfully help protect over 30 bird species versus man” problems such as the impact of communication through land acquisition in 2005 alone? Who could have towers on migrating songbirds beg for realistic solutions and predicted ABC’s leadership in developing collaborative trustworthy organizations like ABC to implement them. bird conservation plans across the United States? And who In summary, bird conservation needs to be led by tough, could have imagined ABC’s effectiveness in building the realistic, smart organizations with broad public support. -

Using Bird Distributions to Assess Extinction Risk and Identify Conservation Priorities In
Using Bird Distributions to Assess Extinction Risk and Identify Conservation Priorities in Biodiversity Hotspots by Natalia Ocampo-Peñuela Nicholas School of the Environment Duke University Date: _________________ Approved: ___________________________ Stuart L. Pimm, Supervisor ___________________________ Jennifer Swenson ___________________________ Nicholas Haddad ___________________________ John R. Poulsen Dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Duke University 2016 ABSTRACT Using Bird Distributions to Assess Extinction Risk and Identify Conservation Priorities in Biodiversity Hotspots by Natalia Ocampo-Peñuela Nicholas School of the Environment Duke University Date: _______________ Approved: 1 ___________________________ Stuart L. Pimm, Supervisor ___________________________ Jennifer Swenson ___________________________ Nicholas Haddad ___________________________ John Poulsen An abstract of a dissertation submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Graduate School of Duke University 2016 2 Copyright by Natalia Ocampo-Peñuela 2016 Abstract Habitat loss, fragmentation, and degradation threaten the World’s ecosystems and species. These, and other threats, will likely be exacerbated by climate change. Due to a limited budget for conservation, we are forced to prioritize a few areas over others. These places are selected based on their uniqueness and vulnerability. One of the most famous examples is the biodiversity hotspots: areas where large quantities of endemic species meet alarming rates of habitat loss. Most of these places are in the tropics, where species have smaller ranges, diversity is higher, and ecosystems are most threatened. Species distributions are useful to understand ecological theory and evaluate iv extinction risk. Small-ranged species, or those endemic to one place, are more vulnerable to extinction than widely distributed species. -

Bird Calls November 2005 Bird Calls November 2005
Vol. 9, No. 3 BBIRDIRD CCALLSALLS November 2005 Avian Influenza Spreads: Role of Wild Birds Debated IN THIS ISSUE Endangered Species Act ecent outbreaks of the Transmission from human to human Faces Extinction? Bill passed avian influenza type ‘A’ has only been recorded in very rare by House weakens ESA protec- R virus, known as H5N1 or cases, and in those cases the disease tions. See story page 3. “avian flu” are causing worldwide has not progressed past the second concern. All subtypes of type ‘A’ individual. Unless the virus mutates Hurricanes Pound Gulf influenza viruses occur naturally in to be readily transmissible from Coast—Sixteen refuges in wild birds, particu- human to human, Louisiana, Mississippi, and larly waterfowl, most the risk of a major Alabama temporarily closed. causing little or no human outbreak is See story page 5. threat to the health low. Most human of birds or people. infections currently However, H5N1 is respond to drugs an especially viru- commonly used to lent and contagious treat human influ- strain, and was first enza strains, though recorded in domestic resistance to those poultry in Asia. It has American Bird Conservancy strongly drugs has been re- since spread to some opposes the culling of wild birds, such as corded in Vietnam these Brant, in response to H5N1. Photo: wild birds in Asia Gary Smyle. and may become a and Eastern Europe. more significant fac- It has also infected tor in the future. more than 100 people and caused ABC’s Position the deaths of 61 of them. Almost all See page 5 for photo caption.