Productivity and Physical Condition of White-Tailed Deer in New Hampshire
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Army Civil Works Program Fy 2020 Work Plan - Operation and Maintenance
ARMY CIVIL WORKS PROGRAM FY 2020 WORK PLAN - OPERATION AND MAINTENANCE STATEMENT OF STATEMENT OF ADDITIONAL LINE ITEM OF BUSINESS MANAGERS AND WORK STATE DIVISION PROJECT OR PROGRAM FY 2020 PBUD MANAGERS WORK PLAN ADDITIONAL FY2020 BUDGETED AMOUNT JUSTIFICATION FY 2020 ADDITIONAL FUNDING JUSTIFICATION PROGRAM PLAN TOTAL AMOUNT AMOUNT 1/ AMOUNT FUNDING 2/ 2/ Funds will be used for specific work activities including AK POD NHD ANCHORAGE HARBOR, AK $10,485,000 $9,685,000 $9,685,000 dredging. AK POD NHD AURORA HARBOR, AK $75,000 $0 Funds will be used for baling deck for debris removal; dam Funds will be used for commonly performed O&M work. outlet channel rock repairs; operations for recreation visitor ENS, FDRR, Funds will also be used for specific work activities including AK POD CHENA RIVER LAKES, AK $7,236,000 $7,236,000 $1,905,000 $9,141,000 6 assistance and public safety; south seepage collector channel; REC relocation of the debris baling area/construction of a baling asphalt roads repairs; and, improve seepage monitoring for deck ($1,800,000). Dam Safety Interim Risk Reduction measures. Funds will be used for specific work activities including AK POD NHS DILLINGHAM HARBOR, AK $875,000 $875,000 $875,000 dredging. Funds will be used for dredging environmental coordination AK POD NHS ELFIN COVE, AK $0 $0 $75,000 $75,000 5 and plans and specifications. Funds will be used for specific work activities including AK POD NHD HOMER HARBOR, AK $615,000 $615,000 $615,000 dredging. Funds are being used to inspect Federally constructed and locally maintained flood risk management projects with an emphasis on approximately 11,750 of Federally authorized AK POD FDRR INSPECTION OF COMPLETED WORKS, AK 3/ $200,000 $200,000 and locally maintained levee systems. -

Official List of Public Waters
Official List of Public Waters New Hampshire Department of Environmental Services Water Division Dam Bureau 29 Hazen Drive PO Box 95 Concord, NH 03302-0095 (603) 271-3406 https://www.des.nh.gov NH Official List of Public Waters Revision Date October 9, 2020 Robert R. Scott, Commissioner Thomas E. O’Donovan, Division Director OFFICIAL LIST OF PUBLIC WATERS Published Pursuant to RSA 271:20 II (effective June 26, 1990) IMPORTANT NOTE: Do not use this list for determining water bodies that are subject to the Comprehensive Shoreland Protection Act (CSPA). The CSPA list is available on the NHDES website. Public waters in New Hampshire are prescribed by common law as great ponds (natural waterbodies of 10 acres or more in size), public rivers and streams, and tidal waters. These common law public waters are held by the State in trust for the people of New Hampshire. The State holds the land underlying great ponds and tidal waters (including tidal rivers) in trust for the people of New Hampshire. Generally, but with some exceptions, private property owners hold title to the land underlying freshwater rivers and streams, and the State has an easement over this land for public purposes. Several New Hampshire statutes further define public waters as including artificial impoundments 10 acres or more in size, solely for the purpose of applying specific statutes. Most artificial impoundments were created by the construction of a dam, but some were created by actions such as dredging or as a result of urbanization (usually due to the effect of road crossings obstructing flow and increased runoff from the surrounding area). -

New England District Team Commemorates Surry Mountain Lake Dam's 75Th Anniversary by Ann Marie R
New England District team commemorates Surry Mountain Lake Dam's 75th anniversary By Ann Marie R. Harvie, USACE New England District For the last 75 years, Surry Mountain Lake Dam in Surry, New Hampshire has stood at the ready to protect New Hampshire residents from flooding. The District team members who operate the project held a 75th anniversary event on October 1 from 10 a.m. to 1 p.m., to commemorate the opening of the dam. “Among the participants that came to the event was a gentleman that worked at Surry Dam in the late 1940’s and early 1950’s,” said Park Ranger Eric Chouinard. “He shared some of his stories and experiences with us.” During the event, Chouinard and Park Ranger Alicia Lacrosse each gave a presentation. “The first was a history presentation,” said Chouinard. “I discussed life in the small town of Surry before the dam’s construction, a brief overview of the history of the U.S. Army Corps of Engineers, the highly important Flood Control Act of 1936 which paved the way for the construction of Surry Dam, the reasoning behind why the town of Surry was chosen as the location for a flood control dam as opposed to other locations in Cheshire County, a brief history with pictures of the flood of 1936 and the hurricane of 1938, which both contributed to the construction of the Surry Dam.” Chouinard’s presentation also featured many historical construction photos. A presentation on invasive plants was given by Lacrosse. “The invasive presentation identified many of the species of local interest, such as Glossy Buckthorn, Japanese Knotweed, Autumn Olive and Eurasian Milfoil,” said Chouinard. -

White Mountain National Forest TTY 603 466-2856 Cover: a Typical Northern Hardwood Stand in the Mill Brook Project Area
Mill Brook United States Department of Project Agriculture Forest Final Service Eastern Environmental Assessment Region Town of Stark Coos County, NH Prepared by the Androscoggin Ranger District November 2008 For Information Contact: Steve Bumps Androscoggin Ranger District 300 Glen Road Gorham, NH 03581 603 466-2713 Ext 227 White Mountain National Forest TTY 603 466-2856 Cover: A typical northern hardwood stand in the Mill Brook project area. This document is available in large print. Contact the Androscoggin Ranger District 603-466-2713 TTY 603-466-2856 The U.S. Department of Agriculture (USDA) prohibits discrimination in all its programs and activi- ties on the basis of race, color, national origin, sex, religion, age, disability, political beliefs, sexual orientation, and marital or family status. (Not all prohibited bases apply to all programs.) Persons with disabilities who require alternative means for communication of program information (Braille, large print, audiotape, etc.) should contact USDA’s TARGET Center at (202) 720-2600 (voice and TDD). To file a complaint of discrimination, write USDA, Director, Office of Civil Rights, Room 326-W, Whitten Building, 1400 Independence Avenue, SW, Washington, DC 20250-9410 or call (202) 720-5964 (voice and TDD). USDA is an equal opportunity provider and employer. Printed on Recycled Paper Mill Brook Project — Environmental Assessment Contents Chapter 1: Purpose and Need......................................................5 1.0 Introduction...............................................................5 1.1 Purpose of the Action and Need for Change . .7 1.2 Decision to be Made .......................................................11 1.3 Public Involvement........................................................12 1.4 Issues . .13 1.5 Alternatives Considered but Not Analyzed in Detail ...........................14 Chapter 2. -

NH Bird Records
New Hampshire Bird Records FALL 2016 Vol. 35, No. 3 IN HONOR OF Rob Woodward his issue of New Hampshire TBird Records with its color cover is sponsored by friends of Rob Woodward in appreciation of NEW HAMPSHIRE BIRD RECORDS all he’s done for birds and birders VOLUME 35, NUMBER 3 FALL 2016 in New Hampshire. Rob Woodward leading a field trip at MANAGING EDITOR the Birch Street Community Gardens Rebecca Suomala in Concord (10-8-2016) and counting 603-224-9909 X309, migrating nighthawks at the Capital [email protected] Commons Garage (8-18-2016, with a rainbow behind him). TEXT EDITOR Dan Hubbard In This Issue SEASON EDITORS Rob Woodward Tries to Leave New Hampshire Behind ...........................................................1 Eric Masterson, Spring Chad Witko, Summer Photo Quiz ...............................................................................................................................1 Lauren Kras/Ben Griffith, Fall Fall Season: August 1 through November 30, 2016 by Ben Griffith and Lauren Kras ................2 Winter Jim Sparrell/Katie Towler, Concord Nighthawk Migration Study – 2016 Update by Rob Woodward ..............................25 LAYOUT Fall 2016 New Hampshire Raptor Migration Report by Iain MacLeod ...................................26 Kathy McBride Field Notes compiled by Kathryn Frieden and Rebecca Suomala PUBLICATION ASSISTANT Loon Freed From Fishing Line in Pittsburg by Tricia Lavallee ..........................................30 Kathryn Frieden Osprey vs. Bald Eagle by Fran Keenan .............................................................................31 -

Investigating Tradeoffs Between Flood Control and Ecological Flow Benefits in the Connecticut River Basin Jocelyn Anleitner
University of Massachusetts Amherst ScholarWorks@UMass Amherst Environmental & Water Resources Engineering Civil and Environmental Engineering Masters Projects 5-2014 Investigating Tradeoffs Between Flood Control And Ecological Flow Benefits in the Connecticut River Basin Jocelyn Anleitner Follow this and additional works at: https://scholarworks.umass.edu/cee_ewre Part of the Environmental Engineering Commons Anleitner, Jocelyn, "Investigating Tradeoffs Between Flood Control And Ecological Flow Benefits in the onneC cticut River Basin" (2014). Environmental & Water Resources Engineering Masters Projects. 64. https://doi.org/10.7275/8vfa-s912 This Article is brought to you for free and open access by the Civil and Environmental Engineering at ScholarWorks@UMass Amherst. It has been accepted for inclusion in Environmental & Water Resources Engineering Masters Projects by an authorized administrator of ScholarWorks@UMass Amherst. For more information, please contact [email protected]. INVESTIGATING TRADEOFFS BETWEEN FLOOD CONTROL AND ECOLOGICAL FLOW BENEFITS IN THE CONNECTICUT RIVER BASIN A Master’s Project Presented By: Jocelyn Anleitner Submitted to the Department of Civil and Environmental Engineering of the University of Massachusetts Amherst in partial fulfillment of the requirements for the degree of Master of Science in Environmental Engineering April 2014 INVESTIGATING TRADEOFFS BETWEEN FLOOD CONTROL AND ECOLOGICAL FLOW BENEFITS IN THE CONNECTICUT RIVER BASIN A Master's Project Presented by JOCELYN ANLEITNER Approved as to style and content by: ~~tor Civil and Environmental Engineering Department ACKNOWLEDGEMENTS I would like to thank The Nature Conservancy (TNC) for funding this work through the Connecticut River Project. This work would not have been possible without, not only their funding, but the support and expertise they have provided. -

Yankee Engineer Volume 41, No
Yankee Voices..................................2 Commander's Corner.....................3 Cape Cod Forrest Knowles...............................4 Patchogue Canal Joe Colucci retires............................5 River Rescues Dredging Up the Past......................8 Page 6 Page 7 US Army Corps of Engineers New England District Yankee Engineer Volume 41, No. 11 August 2006 Reservoirs too small, too shallow Corps of Engineers bans tube kiting at its federal recreation area lakes in New England for safety by Timothy Dugan safety of the public to ban the use of Middlebury (Route 63); Mansfield Public Affairs tube kites, or inflatable flying water- Hollow Lake in Mansfield (Route 6 or craft, at all Corps-managed federal Route 195); Northfield Brook Lake in The U.S. Army Corps of Engi- recreational projects in New England. Thomaston (Route 254); Thomaston neers, New England District issued a Federal projects managed by the Dam in Thomaston (Route 222); and ban as of July 28 on tube kiting at its 31 Corps are located in the following ar- West Thompson Lake in Thompson federal recreation flood control reser- eas. (Route 12). voir projects in New England. In Connecticut: Black Rock Lake In Massachusetts: Barre Falls Dam Signs will be posted detailing the in Thomaston (Route 109); Colebrook in Barre and Hubbardston (Route 62); prohibition. Most of the reservoirs are River Lake in Colebrook (Route 8); Birch Hill Dam in South Royalston too small and too shallow to support any Hancock Brook Lake in Plymouth (Route 68); Buffumville Lake in type of speed boating use. (Waterbury Road); Hop Brook Lake in Charlton (off Route 12); Cape Cod Of the seven lakes Canal in Buzzards Bay (I-195 where the Corps allows boat from Providence and Route 3 operation at speeds that from Boston); Charles River would support tube kites, the Natural Valley Storage Area lakes are not of sufficient in Eastern Massachusetts; size and depth to allow the Conant Brook Dam in Monson activity and ensure public (off Route 32 on Monson- safety. -

Winter 2016-17 Vol. 35 No. 4
New Hampshire Bird Records WINTER 2016-17 Vol. 35, No. 4 IN MEMORY OF Polly Perry his issue of New Hampshire Bird TRecords with its color cover is NEW HAMPSHIRE BIRD RECORDS sponsored by NH Audubon in memory VOLUME 35, NUMBER 4 WINTER 2016-17 of Polly Perry, a volunteer and longtime supporter of the organization. Polly MANAGING EDITOR loved birds and was passionate about Rebecca Suomala environmental education, providing 603-224-9909 X309, [email protected] annual camperships for children in need. Her bequest will help NH TEXT EDITOR Dan Hubbard Audubon continue this work. SEASON EDITORS Eric Masterson, Spring Chad Witko, Summer Lauren Kras/Ben Griffith, Fall In This Issue Jim Sparrell/Katherine Towler, Winter From the Editor, Welcome Jim and Katherine ...........................................................................1 LAYOUT Photo Quiz ...............................................................................................................................1 Kathy McBride Thank You to Donors ................................................................................................................2 PUBLICATION ASSISTANT Winter Season: December 1, 2016 through February 28, 2017 Kathryn Frieden by Jim Sparrell and Katherine Towler ...................................................................................3 ASSISTANTS Christmas Bird Count Summary & Map by David Deifik.......................................................16 Jeannine Ayer, Zeke Cornell, David Deifik, Elizabeth Levy, 117th Christmas Bird Count -
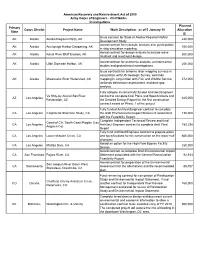
Primary State Corps District Project Name Work Description
American Recovery and Reinvestment Act of 2009 Army Corps of Engineers - Civil Works Investigations Planned Primary Corps District Project Name Work Description - as of 5 January 10 Allocation State ($) Issue contract for State of Alaska Regional Harbor AK Alaska Alaska Regional Ports, AK 240,000 Development Study Award contract for hydraulic analysis and participation AK Alaska Anchorage Harbor Deepening, AK 150,000 in ship simulation modeling. Award contract for design analysis to include wave AK Alaska Kenai River Bluff Erosion, AK 300,000 hindcast and revetment design Award contract for economic analysis, environmental AK Alaska Little Diomede Harbor, AK 250,000 studies and geotechnical investigations Issue contracts for airborne laser mapping surveys in conjunction with US Geologic Survey, wetlands AK Alaska Matanuska River Watershed, AK mapping in conjunction with Fish and Wildlife Service, 372,000 wetlands delineation assessment, and data gap analysis. Fully obligate incrementally funded Architect/Engineer Va Shly-Ay Akimel Salt River contract to complete final Plans and Specifications and AZ Los Angeles 645,000 Restoration, AZ the Detailed Design Report for the first construction contract award on Phase 1 of the project. Fully funded Architect/Engineer contract to complete CA Los Angeles Carpinteria Shoreline Study, CA the draft Environmental Impact Statement associated 130,000 with the Feasibility Report. Complete Independent Technical Review and fund Coast of CA, South Coast Region (Los CA Los Angeles Architect Engineer contract to complete draft Final 162,258 Angeles Co) Report Fully fund Architect/Engineer contract to prepare plans CA Los Angeles Lower Mission Creek, CA and specifications for the construction on the lower half 600,000 of project Award an option for the High Flow Bypass Facility CA Los Angeles Matilija Dam, CA 250,000 Design. -

Northfield and Warwick Community Resilience Building and Hazard Mitigation Regional Plan
DRAFT Northfield and Warwick Community Resilience Building and Hazard Mitigation Regional Plan Adopted by the Northfield and Warwick Select Boards on xx, xx Prepared by Northfield and Warwick Core Teams (Local Planning Team) and Franklin Regional Council of Governments 12 Olive Street, Suite 2 Greenfield, MA 01301 (413) 774-3167 www.frcog.org This project was funded by a grant received from the Massachusetts Executive Office of Energy and Environmental Affairs’ (EEA) Municipal Vulnerability Preparedness Program (MVP). Acknowledgements The Northfield and Warwick Select Boards thank the Northfield and Warwick Core Team (Local Planning Team) for their work on this project. ___________________________ The Northfield and Warwick Select Boards offer thanks to the Massachusetts Emergency Management Agency (MEMA) for developing the 2018 Massachusetts Hazard Mitigation and Climate Adaptation Plan, which served as a resource for this plan. Technical assistance was provided by staff of the Franklin Regional Council of Governments. Peggy Sloan, Director of Planning & Development Kimberly Noake MacPhee, Land Use & Natural Resources Program Manager Alyssa Larose, Senior Land Use & Natural Resources Planner Helena Farrell, Land Use & Natural Resources Planner Allison Gage, Land Use & Natural Resources Planner Alexander Sylvain, Emergency Preparedness Program Assistant Ryan Clary, Senior GIS Specialist Northfield and Warwick Community Resilience Building and Hazard Mitigation Regional Plan i Contents 1 PLANNING PROCESS ............................................................................................................................. -

A Land Conservation Plan for the Ashuelot River Watershed
A Land Conservation Plan for the Ashuelot River Watershed July 2004 Written and edited by Mark Zankel, The Nature Conservancy With contributions from: Doug Bechtel, The Nature Conservancy Brian Hotz, Society for the Protection of New Hampshire Forests Kristen Grubbs and Richard Ober, Monadnock Conservancy Jeff Porter, Southwest Region Planning Commission GIS mapping by Lora Gerard, The Nature Conservancy GAP status analysis research by Jenny Tollefson Developed though a partnership of: The Nature Conservancy Monadnock Conservancy Society for the Protection of New Hampshire Forests Southwest Region Planning Commission Generous funding support provided by: New Hampshire Dept. of Environmental Services Watershed Assistance Program Ashuelot Watershed Conservation Plan Cover Photos: Freshwater Mussels Bald Eagle Cross-country Skier Photo © Jon Golden Photo by Eric Photo by Eric Aldrich Aldrich Beaver Meadow Ashuelot River Photo by Bill Nichols Photo by Eric Aldrich Re-Prints: Copies of this plan may be purchased from The Nature Conservancy for $40 by contacting: The Nature Conservancy 22 Bridge Street, 4th Floor Concord, NH 03301 (603) 224-5853 Permission is required for reproduction of any part of this publication. Copyright 2004, TNC Table of Contents Acknowledgements Executive Summary……………………………………………………………………………………….. i Vision Statement…………………………………………………………………………………………… 1 Section 1: Background……………………………………………………………………………………. 4 Why Develop a Land Conservation Plan for the Ashuelot River watershed ….…………… 4 Purposes of this Plan …………………………………………………………………………………. -

Fall 1999 Vol. 18 No. 3
New Hampshire Bird Records Fall 1999 Vol. 18, No. 3 About the Cover by Rebecca Suomala Black-throated Gray Warbler seen on Star Island on September 21 and 22 A represents the first documented record for this species in New Hampshire. It was discovered feeding in the juniper trees by the cemetery on the southwest corner of the island and stayed within this small area. Despite our hopes and pleas, it never came close to our mist nets (see page 39), and, contrary to reports published elsewhere, we did not band it. Unfortunately, the New Hampshire Rare Bird Alert mistakenly reported that we had banded the bird, and this was picked up by others. I apologize for the confusion. The Black-throated Gray Warbler is a western species that does not breed east of Colorado and New Mexico. It is a handsome black-and-white bird, similar to a Blackpoll Warbler but with a black bib and cheek patch and lovely gray back. The yellow spot between the bill and eye is tiny but diagnostic, if you can see it. We had ample opportunity to observe the bird on Star Island as it fed close by. A large sea swell cancelled all ferry boats, and when good weather arrived, the bird had departed. Those of us lucky enough to be already on the island were the only ones able to see this bird. My photographs, while acceptable for documentation, were not of suitable quality for the cover of New Hampshire Bird Records – in anticipation of photographing birds in the hand, I did not bring a long lens with me to Star, and a photograph of a tiny spot was the sad result.