Nanocrystal Facet Modulation to Enhance Transferrin Binding And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Nanoparticles, Nanocrystals, and Quantum Dots What They Are, Why They’Re Interesting, and What We Can Do with Them J
Nanoparticles, nanocrystals, and quantum dots What they are, why they’re interesting, and what we can do with them J. Nadeau, Department of Biomedical Engineering [email protected] Colloidal nanocrystals of different materials… …And different geometries From: Science. 2005 January 28; 307(5709): 538544. Medieval Nanotechnology! The colors in some stained- glass windows from medieval cathedrals are probably due to nanocrystals of compouds of Zn, Cd, S, and Se. History of nanoparticles 1980 Ekimov observed quantum confinement on a sample of glass containing PbS. 1982 Brus L.’s group conducted CdS colloid preparation and investigation of band-edge luminescence properties. 1993 Murray C., Norris D., Bawendi M., Synthesis and Characterization of Nearly Monodisperse CdE (E=S, Se, Te) Semiconductor Nano- crystallites. 1995 Hines M., Guyot-Sionnest P., reported synthesis and Characterization of Strongly Luminescent ZnS-Capped CdSe Nanocrystals 1998 Alivisatos and Nie independently reported Bio-application for core shell dots. 2001 Nie’s group described Quantum dot-tagged microbeads for multiplexed optical coding of biomolecules. 2003 T. Sargent at UOT observed electroluminescence spanning 1000 – 1600 nm originating from PbS nanocrystals embedded in a polymer matrix. What is a quantum dot? • Synthesis • Quantum mechanics • Optical properties WhatWhat isis itit goodgood for?for? ••InterestingInteresting physicsphysics ••ApplicationsApplications inin optoelectronicsoptoelectronics ••ApplicationsApplications inin biologybiology Synthesis Quick -

Common Gene Polymorphisms Associated with Thrombophilia
Chapter 5 Common Gene Polymorphisms Associated with Thrombophilia Christos Yapijakis, Zoe Serefoglou and Constantinos Voumvourakis Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61859 Abstract Genetic association studies have revealed a correlation between DNA variations in genes encoding factors of the hemostatic system and thrombosis-related disease. Certain var‐ iant alleles of these genes that affect either gene expression or function of encoded protein are known to be genetic risk factors for thrombophilia. The chapter presents the current genetics and molecular biology knowledge of the most important DNA polymorphisms in thrombosis-related genes encoding coagulation factor V (FV), coagulation factor II (FII), coagulation factor XII (FXII), coagulation factor XIII A1 subunit (FXIIIA1), 5,10- methylene tetrahydrofolate reductase (MTHFR), serpine1 (SERPINE1), angiotensin I-con‐ verting enzyme (ACE), angiotensinogen (AGT), integrin A2 (ITGA2), plasma carboxypeptidase B2 (CPB2), platelet glycoprotein Ib α polypeptide (GP1BA), thrombo‐ modulin (THBD) and protein Z (PROZ). The molecular detection methods of each DNA polymorphism is presented, in addition to the current knowledge regarding its influence on thrombophilia and related thrombotic events, including stroke, myocardial infarction, deep vein thrombosis, spontaneous abortion, etc. In addition, best thrombosis prevention strategies with a combination of genetic counseling and molecular testing are discussed. Keywords: Thrombophilia, coagulation -

Charge Transport in Semiconductors Assembled from Nanocrystal Quantum Dots
ARTICLE https://doi.org/10.1038/s41467-020-16560-7 OPEN Charge transport in semiconductors assembled from nanocrystal quantum dots Nuri Yazdani1, Samuel Andermatt2, Maksym Yarema 1, Vasco Farto1, Mohammad Hossein Bani-Hashemian 2, Sebastian Volk1, Weyde M. M. Lin 1, Olesya Yarema 1, ✉ Mathieu Luisier 2 & Vanessa Wood 1 The potential of semiconductors assembled from nanocrystals has been demonstrated for a 1234567890():,; broad array of electronic and optoelectronic devices, including transistors, light emitting diodes, solar cells, photodetectors, thermoelectrics, and phase change memory cells. Despite the commercial success of nanocrystal quantum dots as optical absorbers and emitters, applications involving charge transport through nanocrystal semiconductors have eluded exploitation due to the inability to predictively control their electronic properties. Here, we perform large-scale, ab initio simulations to understand carrier transport, generation, and trapping in strongly confined nanocrystal quantum dot-based semiconductors from first principles. We use these findings to build a predictive model for charge transport in these materials, which we validate experimentally. Our insights provide a path for systematic engineering of these semiconductors, which in fact offer previously unexplored opportunities for tunability not achievable in other semiconductor systems. 1 Materials and Device Engineering Group, Department of Information Technology and Electrical Engineering, ETH Zurich, 8092 Zurich, Switzerland. 2 Nano ✉ TCAD Group, Department -

A Differential Protein Solubility Approach for the Depletion of Highly Abundant Proteins in Plasma Using Ammonium Sulfate Ravi Chand Bollineni1, 2*, Ingrid J
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2015 A differential protein solubility approach for the depletion of highly abundant proteins in plasma using ammonium sulfate Ravi Chand Bollineni1, 2*, Ingrid J. Guldvik3, Henrik Gronberg4, Fredrik Wiklund4, Ian G. Mills3, 5, 6 and Bernd Thiede1, 2 1Department of Biosciences, University of Oslo, Oslo, Norway 2Biotechnology Centre of Oslo, University of Oslo, Oslo, Norway 3Centre for Molecular Medicine Norway (NCMM), University of Oslo and Oslo University Hospitals, Norway 4Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden 5Department of Cancer Prevention, Oslo University Hospitals, Oslo, Norway 6Department of Urology, Oslo University Hospitals, Oslo, Norway Keywords: ammonium sulfate, blood, depletion, plasma, protein precipitation *To whom the correspondence should be addressed: Ravi Chand Bollineni, Department of Biosciences, University of Oslo, P.O. Box 1066 Blindern, 0316 Oslo, Norway, Tel.: +47-22840512; Fax +47-22840501; E-mail: [email protected] Supplementary information Figure S1: SDS-PAGE analysis of serum proteins precipitated with the ethanol/sodium acetate (A), TCA/acetone (B) and ammonium sulfate precipitation (C). A) Ethanol/sodium acetate precipitation: (1) pellet obtained after 42% ethanol precipitation and (2) pellet obtained after precipitation of proteins in the supernatant with 0.8M sodium acetate (pH5.7) and (3) proteins left over in the supernatant. B) Serum proteins are precipitated with 10% TCA/acetone (1) and (2) proteins left over in the supernatant. C) Serum proteins are precipitated with increasing ammonium sulfate concentrations 15% (1), 25% (2), 35% (3), 40% (4), 45% (5), 50% (6) and total serum (7). -

RSC NC-2Edn Contents 23..36
Contents List of Acronyms xxxvii Teaching (Nano) Materials xli Learning (Nano) Materials xliii Guessing (Nano) Materials xliv About the Authors xlv Acknowledgements l Nanofood for Thought–Thinking about Nanochemistry, Nanoscience, Nanotechnology and Nanosafety lii Chapter 1 Nanochemistry Basics 3 1.1 The Roots of Nanochemistry in Materials Chemistry 3 1.2 Synthesis of Materials and Nanomaterials 5 1.3 Materials Self-Assembly 9 1.4 Big Bang to the Universe 10 1.5 Why Nano? 10 1.6 What Do we Mean by Large and Small Nanomaterials? 11 1.7 Do it Yourself Quantum Mechanics 12 1.8 What is Nanochemistry? 13 1.9 Molecular vs. Materials Self-Assembly 13 1.10 What is Hierarchical Assembly? 14 Nanochemistry: A Chemical Approach to Nanomaterials By Geoffrey A. Ozin, Andre´C. Arsenault and Ludovico Cademartiri r Geoffrey A. Ozin, Andre´C. Arsenault and Ludovico Cademartiri 2009 Published by the Royal Society of Chemistry, www.rsc.org xxiii xxiv Contents 1.11 Directing Self-Assembly 15 1.12 Supramolecular Vision 15 1.13 Genealogy of Self-Assembling Materials 16 1.14 Unlocking the Key to Porous Solids 19 1.15 Learning from Biominerals – Form is Function 22 1.16 Can You Curve a Crystal? 24 1.17 Patterns, Patterns Everywhere 25 1.18 Synthetic Creations with Natural Form 26 1.19 Two-Dimensional Assemblies 28 1.20 SAMs and Soft Lithography 31 1.21 Clever Clusters 32 1.22 Extending the Prospects of Nanowires 34 1.23 Coercing Colloids 35 1.24 Mesoscale Self-Assembly 38 1.25 Materials Self-Assembly of Integrated Systems 40 References 41 Nanofood for Thought – -

Gelation of Plasmonic Metal Oxide Nanocrystals by Polymer-Induced Depletion Attractions
Gelation of plasmonic metal oxide nanocrystals by polymer-induced depletion attractions Camila A. Saez Cabezasa, Gary K. Onga,b, Ryan B. Jadricha, Beth A. Lindquista, Ankit Agrawala, Thomas M. Trusketta,c,1, and Delia J. Millirona,1 aMcKetta Department of Chemical Engineering, The University of Texas at Austin, Austin, TX 78712; bDepartment of Materials Science and Engineering, University of California, Berkeley, CA 94720; and cDepartment of Physics, The University of Texas at Austin, Austin, TX 78712 Edited by Catherine J. Murphy, University of Illinois at Urbana–Champaign, Urbana, IL, and approved July 26, 2018 (received for review April 22, 2018) Gelation of colloidal nanocrystals emerged as a strategy to preserve silver fuse into wire-like networks when assembled into self-supported inherent nanoscale properties in multiscale architectures. However, nanoparticle gels, obliterating the LSPR optical response char- available gelation methods to directly form self-supported nano- acteristic of the isolated nanoparticles (22, 23). We sought a new crystal networks struggle to reliably control nanoscale optical strategy for gelation using physical bonding interactions, which phenomena such as photoluminescence and localized surface plas- we hypothesized could maintain the discrete morphology of LSPR- mon resonance (LSPR) across nanocrystal systems due to processing active metal oxides intact, to target LSPR-active nanocrystal gels. variabilities. Here, we report on an alternative gelation method based Our approach is not specific to the chemistry of the nanocrystals on physical internanocrystal interactions: short-range depletion at- employed and could potentially enable a broad class of gels as- tractions balanced by long-range electrostatic repulsions. The latter sembled from diverse nanoscale components capable of reflect- are established by removing the native organic ligands that passivate ing their individual properties. -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Quantitative Theories of Nanocrystal Growth Processes
QUANTITATIVE THEORIES OF NANOCRYSTAL GROWTH PROCESSES Michael D. Clark Submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Columbia University Graduate School of Arts and Sciences COLUMBIA UNIVERSITY 2013 © 2012 Michael D. Clark All Rights Reserved ABSTRACT Quantitative Theories of Nanocrystal Growth Processes Michael D. Clark Nanocrystals are an important field of study in the 21 st century. Crystallites that are nanometers in size have very different properties from their bulk analogs because quantum mechanical effects become dominant at such small length scales. When a crystallite becomes small enough, the quantum confinement of electrons in the material manifests as a size-dependence of the nanocrystal's properties. Electrical and optical properties such as absorbance, surface plasmon resonance, and photoluminescence are sensitive to the size of the nanocrystal and proffer an array of technological applications for nanocrystals in such fields as biological imaging, laser technology, solar power enhancement, LED modification, chemical sensors, and quantum computation. The synthesis of size-controlled nanocrystals is critical to using nanocrystal in applications for their size-dependent properties. The development of nanocrystal synthesis techniques has been its own entire field of study for two decades or more, and several successes have established novel, utilitarian protocols for the mass-production of nanocrystals with controlled size and very low polydispersity. However, the experimental successes are generally poorly understood and no theoretical framework exists to explain the dynamics of these processes and how to better control or optimize them. It is the goal of this thesis to develop novel theories of nanocrystal synthesis processes to describe these phenomena in theoretical detail and extract meaningful correlations and driving forces that provide the necessary insight to improve the technology and enhance our understanding of nanocrystal growth. -
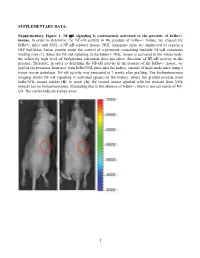
Activation of NF-Κb Signaling Promotes Prostate Cancer Progression in the Mouse and Predicts Poor Progression and Death in Pati
SUPPLEMENTARY DATA: Supplementary Figure 1. NF-B signaling is continuously activated in the prostate of - mouse. In order to determine the NF- '- mouse, we crossed the '- mice with NGL, a NF-B reporter mouse. NGL transgenic mice are engineered to express a GFP/luciferase fusion protein under the control of a promoter containing multiple NF-B consensus binding sites (1). Since the NF-'- NGL mouse is activated in the whole body, the relatively high level of background activation does not allow detection of NF- prostate. Therefore, in order to determine the NF-the '- mouse, we grafted the prostates from 'o the kidney capsule of male nude mice using a tissue rescue technique. NF-B activity was measured at 7 weeks after grafting. The bioluminescence imaging shows NF-B signaling is activated (green) in the kidney, where the grafted prostate from ' use resides (B). In panel (A), the control mouse (grafted with the prostate from NGL mouse) has no bioluminescence, illustrating that in the absence of '-, there is not activation of NF- B. The circles indicate kidney areas. 1 Supplementary Figure 2. NF-B signaling activated in the prostate of Myc/IB bigenic mouse. The prostates from Myc alone (Myc) and bigenic (Myc/IB) mice were harvested at 6 months of age. Activation of NF-B signaling in the prostate was determined by IHC staining of p65-pho antibody. 2 Supplementary Figure 3. Continuous activation of NF-B signaling promotes PCa progression in the Hi-Myc transgenic mouse. The prostates from Myc alone (Myc) and bigeneic (Myc/IB) mice were harvested at 6 months of age. -

Carboxypeptidase B2 (CPB2) Human Shrna Lentiviral Particle (Locus ID 1361) Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TL305252V Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Product data: Product Type: shRNA Lentiviral Particles Product Name: Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Locus ID: 1361 Synonyms: CPU; PCPB; TAFI Vector: pGFP-C-shLenti (TR30023) Format: Lentiviral particles RefSeq: NM_001278541, NM_001872, NM_016413, NM_001872.1, NM_001872.2, NM_001872.3, NM_001872.4, NM_016413.1, NM_016413.2, NM_016413.3, NM_001278541.1, BC007057, BC007057.1, NM_001872.5 Summary: Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by trypsin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis. Polymorphisms have been described for this gene and its promoter region. Alternate splicing results in multiple transcript variants. [provided by RefSeq, Jun 2013] shRNA Design: These shRNA constructs were designed against multiple splice variants at this gene locus. To be certain that your variant of interest is targeted, -

How to Dope a Semiconductor Nanocrystal? Uri Banin Institute Of
Abstract #2152, 224th ECS Meeting, © 2013 The Electrochemical Society How to dope a semiconductor nanocrystal? Uri Banin Institute of Chemistry and the Center for Nanoscience and Nanotechnology, The Hebrew University of Jerusalem, Jerusalem 91904, Israel Doping of bulk semiconductors, the process of intentional introduction of impurity atoms into a crystal discovered back in the 1940s, is a key enabling route for tuning their properties. Its introduction allowed the wide-spread application of semiconductors in electronic and electro- optic components. Controlling the size and dimensionality of semiconductor structures is an additional powerful way to tune their properties via quantum confinement effects. In this respect, colloidal semiconductor nanocrystals have emerged as a family of materials with size dependent optical and electronic properties that have attracted significant attention due to their unique attributes and potential applications. Impurity doping in such colloidal nanocrystals still remains an open challenge. From the Figure 1: Effects of doping in semiconductor synthesis side, the introduction of a few impurity atoms nanocrystals. Shown is a sketch for n-doped into a nanocrystal which contains only a few hundred nanocrystal quantum dot with confined energy atoms may lead to their expulsion to the surface or levels, red and green lines correspond to the compromise the crystal structure. From a physical QD and impurity levels, respectively. Left: viewpoint, impurities inherently create a heavily doped The level diagram for a single impurity nanocrystal under strong quantum confinement, and the effective mass model, Right: Impurity levels electronic and optical properties in such circumstances are develop into impurity bands as the number of still unresolved. -

Electronic Supplementary Material (ESI) for Analyst. This Journal Is © the Royal Society of Chemistry 2020
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2020 Table S1.