Proteomics Analysis Reveals a Th17-Prone Cell Population in Pre-Symptomatic Graft- Versus-Host Disease
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Common Gene Polymorphisms Associated with Thrombophilia
Chapter 5 Common Gene Polymorphisms Associated with Thrombophilia Christos Yapijakis, Zoe Serefoglou and Constantinos Voumvourakis Additional information is available at the end of the chapter http://dx.doi.org/10.5772/61859 Abstract Genetic association studies have revealed a correlation between DNA variations in genes encoding factors of the hemostatic system and thrombosis-related disease. Certain var‐ iant alleles of these genes that affect either gene expression or function of encoded protein are known to be genetic risk factors for thrombophilia. The chapter presents the current genetics and molecular biology knowledge of the most important DNA polymorphisms in thrombosis-related genes encoding coagulation factor V (FV), coagulation factor II (FII), coagulation factor XII (FXII), coagulation factor XIII A1 subunit (FXIIIA1), 5,10- methylene tetrahydrofolate reductase (MTHFR), serpine1 (SERPINE1), angiotensin I-con‐ verting enzyme (ACE), angiotensinogen (AGT), integrin A2 (ITGA2), plasma carboxypeptidase B2 (CPB2), platelet glycoprotein Ib α polypeptide (GP1BA), thrombo‐ modulin (THBD) and protein Z (PROZ). The molecular detection methods of each DNA polymorphism is presented, in addition to the current knowledge regarding its influence on thrombophilia and related thrombotic events, including stroke, myocardial infarction, deep vein thrombosis, spontaneous abortion, etc. In addition, best thrombosis prevention strategies with a combination of genetic counseling and molecular testing are discussed. Keywords: Thrombophilia, coagulation -

2007-05 TLE1 Synovial Sarcoma
TLE1 Immunostains in the Diagnosis of Synovial Sarcoma May 2007 by Rodney T. Miller, M.D., Director of Immunohistochemistry The diagnosis of synovial sarcoma can be a chal- lenging task, particularly on small biopsy specimens, as the morphologic features of this tumor can be mimicked by a variety of other neoplasms. This month we call attention to a paper published in the February 2007 edition of the American Journal of Surgical Pathology describing the utility of immu- nostains for TLE1 in the diagnosis of this tumor. TLE immunostains are now available at ProPath. Synovial sarcoma occurs in three morphologic varie- ties: monophasic, biphasic, and poorly differentiated. It has been known for some time that synovial sar- coma is associated with a specific chromosomal H&E (left) and TLE1 immunostain (right) on a monophasic translocation, t(X;18), that results in the fusion of the synovial sarcoma. Note the numerous strongly positive nuclei SYT gene on chromosome 18 to either the SSX1 or on the TLE1 immunostain, a typical feature of this tumor. SSX2 gene on the X chromosome, resulting in the production of a SYT-SSX fusion protein. Identifica- In their study, the authors performed TLE1 immu- tion of this translocation in the appropriate setting is nostains on multiple tissue microarrays using two dif- regarded by many to be diagnostic of synovial sar- ferent antibodies (monoclonal and polyclonal), both coma. However, the methodologies used for this performing in a similar fashion. A total of 693 adult purpose (cytogenetics, fluorescent in situ hybridiza- soft tissue tumors were examined, including 94 cases tion, and reverse-transcriptase polymerase chain re- of synovial sarcoma that had documentation of the t action) are not readily available in many diagnostic (X;18) translocation. -

A Differential Protein Solubility Approach for the Depletion of Highly Abundant Proteins in Plasma Using Ammonium Sulfate Ravi Chand Bollineni1, 2*, Ingrid J
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2015 A differential protein solubility approach for the depletion of highly abundant proteins in plasma using ammonium sulfate Ravi Chand Bollineni1, 2*, Ingrid J. Guldvik3, Henrik Gronberg4, Fredrik Wiklund4, Ian G. Mills3, 5, 6 and Bernd Thiede1, 2 1Department of Biosciences, University of Oslo, Oslo, Norway 2Biotechnology Centre of Oslo, University of Oslo, Oslo, Norway 3Centre for Molecular Medicine Norway (NCMM), University of Oslo and Oslo University Hospitals, Norway 4Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden 5Department of Cancer Prevention, Oslo University Hospitals, Oslo, Norway 6Department of Urology, Oslo University Hospitals, Oslo, Norway Keywords: ammonium sulfate, blood, depletion, plasma, protein precipitation *To whom the correspondence should be addressed: Ravi Chand Bollineni, Department of Biosciences, University of Oslo, P.O. Box 1066 Blindern, 0316 Oslo, Norway, Tel.: +47-22840512; Fax +47-22840501; E-mail: [email protected] Supplementary information Figure S1: SDS-PAGE analysis of serum proteins precipitated with the ethanol/sodium acetate (A), TCA/acetone (B) and ammonium sulfate precipitation (C). A) Ethanol/sodium acetate precipitation: (1) pellet obtained after 42% ethanol precipitation and (2) pellet obtained after precipitation of proteins in the supernatant with 0.8M sodium acetate (pH5.7) and (3) proteins left over in the supernatant. B) Serum proteins are precipitated with 10% TCA/acetone (1) and (2) proteins left over in the supernatant. C) Serum proteins are precipitated with increasing ammonium sulfate concentrations 15% (1), 25% (2), 35% (3), 40% (4), 45% (5), 50% (6) and total serum (7). -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

Characterizing Novel Interactions of Transcriptional Repressor Proteins BCL6 & BCL6B
Characterizing Novel Interactions of Transcriptional Repressor Proteins BCL6 & BCL6B by Geoffrey Graham Lundell-Smith A thesis submitted in conformity with the requirements for the degree of Master of Science Department of Biochemistry University of Toronto © Copyright by Geoffrey Lundell-Smith, 2017 Characterizing Novel Interactions of Transcriptional Repression Proteins BCL6 and BCL6B Geoffrey Graham Lundell-Smith Masters of Science Department of Biochemistry University of Toronto 2016 Abstract B-cell Lymphoma 6 (BCL6) and its close homolog BCL6B encode proteins that are members of the BTB-Zinc Finger family of transcription factors. BCL6 plays an important role in regulating the differentiation and proliferation of B-cells during the adaptive immune response, and is also involved in T cell development and inflammation. BCL6 acts by repressing genes involved in DNA damage response during the affinity maturation of immunoglobulins, and the mis- expression of BCL6 can lead to diffuse large B-cell lymphoma. Although BCL6B shares high sequence similarity with BCL6, the functions of BCL6B are not well-characterized. I used BioID, an in vivo proximity-dependent labeling method, to identify novel BCL6 and BCL6B protein interactors and validated a number of these interactions with co-purification experiments. I also examined the evolutionary relationship between BCL6 and BCL6B and identified conserved residues in an important interaction interface that mediates corepressor binding and gene repression. ii Acknowledgments Thank you to my supervisor, Gil Privé for his mentorship, guidance, and advice, and for giving me the opportunity to work in his lab. Thanks to my committee members, Dr. John Rubinstein and Dr. Jeff Lee for their ideas, thoughts, and feedback during my Masters. -

Analysis of the Indacaterol-Regulated Transcriptome in Human Airway
Supplemental material to this article can be found at: http://jpet.aspetjournals.org/content/suppl/2018/04/13/jpet.118.249292.DC1 1521-0103/366/1/220–236$35.00 https://doi.org/10.1124/jpet.118.249292 THE JOURNAL OF PHARMACOLOGY AND EXPERIMENTAL THERAPEUTICS J Pharmacol Exp Ther 366:220–236, July 2018 Copyright ª 2018 by The American Society for Pharmacology and Experimental Therapeutics Analysis of the Indacaterol-Regulated Transcriptome in Human Airway Epithelial Cells Implicates Gene Expression Changes in the s Adverse and Therapeutic Effects of b2-Adrenoceptor Agonists Dong Yan, Omar Hamed, Taruna Joshi,1 Mahmoud M. Mostafa, Kyla C. Jamieson, Radhika Joshi, Robert Newton, and Mark A. Giembycz Departments of Physiology and Pharmacology (D.Y., O.H., T.J., K.C.J., R.J., M.A.G.) and Cell Biology and Anatomy (M.M.M., R.N.), Snyder Institute for Chronic Diseases, Cumming School of Medicine, University of Calgary, Calgary, Alberta, Canada Received March 22, 2018; accepted April 11, 2018 Downloaded from ABSTRACT The contribution of gene expression changes to the adverse and activity, and positive regulation of neutrophil chemotaxis. The therapeutic effects of b2-adrenoceptor agonists in asthma was general enriched GO term extracellular space was also associ- investigated using human airway epithelial cells as a therapeu- ated with indacaterol-induced genes, and many of those, in- tically relevant target. Operational model-fitting established that cluding CRISPLD2, DMBT1, GAS1, and SOCS3, have putative jpet.aspetjournals.org the long-acting b2-adrenoceptor agonists (LABA) indacaterol, anti-inflammatory, antibacterial, and/or antiviral activity. Numer- salmeterol, formoterol, and picumeterol were full agonists on ous indacaterol-regulated genes were also induced or repressed BEAS-2B cells transfected with a cAMP-response element in BEAS-2B cells and human primary bronchial epithelial cells by reporter but differed in efficacy (indacaterol $ formoterol . -

Hes6 Inhibits Astrocyte Differentiation and Promotes Neurogenesis Through Different Mechanisms
The Journal of Neuroscience, October 25, 2006 • 26(43):11061–11071 • 11061 Cellular/Molecular Hes6 Inhibits Astrocyte Differentiation and Promotes Neurogenesis through Different Mechanisms Sumit Jhas, Sorana Ciura,* Stephanie Belanger-Jasmin,* Zhifeng Dong, Estelle Llamosas, Francesca M. Theriault, Kerline Joachim, Yeman Tang, Lauren Liu, Jisheng Liu, and Stefano Stifani Center for Neuronal Survival, Montreal Neurological Institute, McGill University, Montreal, Quebec, Canada H3A 2B4 The mechanisms regulating the generation of cell diversity in the mammalian cerebral cortex are beginning to be elucidated. In that regard, Hairy/Enhancer of split (Hes) 1 and 5 are basic helix-loop-helix (bHLH) factors that inhibit the differentiation of pluripotent cortical progenitors into neurons. In contrast, a related Hes family member termed Hes6 promotes neurogenesis. It is shown here that knockdown of endogenous Hes6 causes supernumerary cortical progenitors to differentiate into cells that exhibit an astrocytic morphol- ogy and express the astrocyte marker protein GFAP. Conversely, exogenous Hes6 expression in cortical progenitors inhibits astrocyte differentiation. The negative effect of Hes6 on astrocyte differentiation is independent of its ability to promote neuronal differentiation. We also show that neither its proneuronal nor its anti-gliogenic functions appear to depend on Hes6 ability to bind to DNA via the basic arm of its bHLH domain. Both of these activities require Hes6 to be localized to nuclei, but only its anti-gliogenic function depends on two short peptides, LNHLL and WRPW, that are conserved in all Hes6 proteins. These findings suggest that Hes6 is an important regulator of the neurogenic phase of cortical development by promoting the neuronal fate while suppressing astrocyte differentiation. -

Variability in the Degree of Expression of Phosphorylated I B in Chronic
6796 Vol. 10, 6796–6806, October 15, 2004 Clinical Cancer Research Featured Article Variability in the Degree of Expression of Phosphorylated IB␣ in Chronic Lymphocytic Leukemia Cases With Nodal Involvement Antonia Rodrı´guez,1 Nerea Martı´nez,1 changes in the expression profile (mRNA and protein ex- Francisca I. Camacho,1 Elena Ruı´z-Ballesteros,2 pression) and clinical outcome in a series of CLL cases with 2 1 lymph node involvement. Activation of NF-B, as deter- Patrocinio Algara, Juan-Fernando Garcı´a, ␣ 3 5 mined by the expression of p-I B , was associated with the Javier Mena´rguez, Toma´s Alvaro, expression of a set of genes comprising key genes involved in 6 4 Manuel F. Fresno, Fernando Solano, the control of B-cell receptor signaling, signal transduction, Manuela Mollejo,2 Carmen Martin,1 and and apoptosis, including SYK, LYN, BCL2, CCR7, BTK, Miguel A. Piris1 PIK3CD, and others. Cases with increased expression of ␣ 1Molecular Pathology Program, Centro Nacional de Investigaciones p-I B showed longer overall survival than cases with lower Oncolo´gicas, Madrid, Spain; 2Department of Genetics and Pathology, expression. A Cox regression model was derived to estimate 3 Hospital Virgen de la Salud, Toledo, Spain; Department of some parameters of prognostic interest: IgVH mutational Pathology, Hospital General Universitario Gregorio Maran˜o´n, ␣ 4 status, ZAP-70, and p-I B expression. The multivariate Madrid, Spain; Department of Hematology, Hospital Nuestra Sen˜ora analysis disclosed p-IB␣ and ZAP-70 expression as inde- del Prado, Talavera de la Reina, Toledo, Spain; 5Department of Pathology, Hospital Verge de la Cinta, Tortosa, Spain; pendent prognostic factors of survival. -
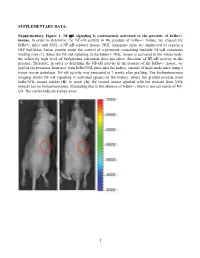
Activation of NF-Κb Signaling Promotes Prostate Cancer Progression in the Mouse and Predicts Poor Progression and Death in Pati
SUPPLEMENTARY DATA: Supplementary Figure 1. NF-B signaling is continuously activated in the prostate of - mouse. In order to determine the NF- '- mouse, we crossed the '- mice with NGL, a NF-B reporter mouse. NGL transgenic mice are engineered to express a GFP/luciferase fusion protein under the control of a promoter containing multiple NF-B consensus binding sites (1). Since the NF-'- NGL mouse is activated in the whole body, the relatively high level of background activation does not allow detection of NF- prostate. Therefore, in order to determine the NF-the '- mouse, we grafted the prostates from 'o the kidney capsule of male nude mice using a tissue rescue technique. NF-B activity was measured at 7 weeks after grafting. The bioluminescence imaging shows NF-B signaling is activated (green) in the kidney, where the grafted prostate from ' use resides (B). In panel (A), the control mouse (grafted with the prostate from NGL mouse) has no bioluminescence, illustrating that in the absence of '-, there is not activation of NF- B. The circles indicate kidney areas. 1 Supplementary Figure 2. NF-B signaling activated in the prostate of Myc/IB bigenic mouse. The prostates from Myc alone (Myc) and bigenic (Myc/IB) mice were harvested at 6 months of age. Activation of NF-B signaling in the prostate was determined by IHC staining of p65-pho antibody. 2 Supplementary Figure 3. Continuous activation of NF-B signaling promotes PCa progression in the Hi-Myc transgenic mouse. The prostates from Myc alone (Myc) and bigeneic (Myc/IB) mice were harvested at 6 months of age. -

RUNX3 Is Multifunctional in Carcinogenesis of Multiple Solid Tumors
Oncogene (2010) 29, 2605–2615 & 2010 Macmillan Publishers Limited All rights reserved 0950-9232/10 $32.00 www.nature.com/onc REVIEW RUNX3 is multifunctional in carcinogenesis of multiple solid tumors LSH Chuang1 and Y Ito1,2,3 1Cancer Biology Program, Cancer Science Institute of Singapore, National University of Singapore, Singapore; 2Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore, Singapore and 3Institute of Molecular and Cell Biology, Singapore The study of RUNX3 in tumor pathogenesis is a rapidly Sgpp1, Ncam1, p21WAF1) (Wotton et al., 2008), the expanding area of cancer research. Functional inactiva- RUNX proteins have been shown to perform distinct tion of RUNX3—through mutation, epigenetic silencing, functions that are dependent on tissue type (Brady and or cytoplasmic mislocalization—is frequently observed in Farrell, 2009). This review explores the dualistic solid tumors of diverse origins. This alone indicates that associations of RUNX3 with cancer with emphasis on RUNX3 inactivation is a major risk factor in tumorigen- the emerging concept of RUNX3 as a multifunctional esis and that it occurs early during progression to suppressor of solid tumors. malignancy. Conversely, RUNX3 has also been described to have an oncogenic function in a subset of tumors. Although the mechanism of how RUNX3 switches from RUNX3 inactivation is prevalent in solid tumors tumor suppressive to oncogenic activity is unclear, this is of clinical relevance with implications for cancer detection Hemizygous deletion of RUNX3 gene and prognosis. Recent developments have significantly The location of RUNX3 at chromosome 1p36, a deletion contributed to our understanding of the pleiotropic tumor hotspot in diverse cancers of epithelial, hematopoietic, suppressive properties of RUNX3 that regulate major and neural origins (Bagchi and Mills, 2008), prompts signaling pathways. -

Carboxypeptidase B2 (CPB2) Human Shrna Lentiviral Particle (Locus ID 1361) Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TL305252V Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Product data: Product Type: shRNA Lentiviral Particles Product Name: Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Locus ID: 1361 Synonyms: CPU; PCPB; TAFI Vector: pGFP-C-shLenti (TR30023) Format: Lentiviral particles RefSeq: NM_001278541, NM_001872, NM_016413, NM_001872.1, NM_001872.2, NM_001872.3, NM_001872.4, NM_016413.1, NM_016413.2, NM_016413.3, NM_001278541.1, BC007057, BC007057.1, NM_001872.5 Summary: Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by trypsin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis. Polymorphisms have been described for this gene and its promoter region. Alternate splicing results in multiple transcript variants. [provided by RefSeq, Jun 2013] shRNA Design: These shRNA constructs were designed against multiple splice variants at this gene locus. To be certain that your variant of interest is targeted, -

TLE1 Corepressor Complex Mediates a Camkinase II␦-Dependent Neurogenic Gene Activation Pathway
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector Cell, Vol. 119, 815–829, December 17, 2004, Copyright ©2004 by Cell Press ActivatingthePARP-1SensorComponentoftheGroucho/ TLE1 Corepressor Complex Mediates a CaMKinase II␦-Dependent Neurogenic Gene Activation Pathway Bong-Gun Ju,1 Derek Solum,1 Eun Joo Song,4 entiate into neurons in response to both extrinsic and Kong-Joo Lee,4 David W. Rose,2 intrinsic cues (reviewed in Bally-Cuif and Hammer- Christopher K. Glass,3 and Michael G. Rosenfeld1,* schmidt [2003]). The commitment of neural stem cells 1Howard Hughes Medical Institute to the neuronal fate and their subsequent differentiation 2 Department of Medicine into neurons are controlled, in part, by mechanisms in- Division of Endocrinology volving the opposing activities of transcription factor 3 Department of Cellular and Molecular Medicine families containing the basic helix-loop-helix (bHLH) do- University of California, San Diego main, the Hairy/Enhancer of split (HES) family proteins Department and School of Medicine that negatively regulate neural differentiation, and other La Jolla, California 92093 bHLH family proteins belonging to the Neurogenin, Ash, 4 Center for Cell Signaling Research and NeuroD subgroups, collectively referred to as Division of Molecular Life Sciences and College proneural proteins (reviewed in Bertrand et al. [2002]; of Pharmacy Ross et al. [2003]). Ewha Womans University The inhibitory bHLH protein, HES1, interacts with Seoul 120-750 groucho (Gro)/transducin like enhancer of split (TLE) Korea corepressor in a manner dependent on its C-terminal WRPW motif and serves in a well-described repressor of MASH1 transcription preventing neuronal differentiation Summary (reviewed in Chen and Courey [2000]).