Common Gene Polymorphisms Associated with Thrombophilia
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Hemoglobin Interaction with Gp1ba Induces Platelet Activation And
ARTICLE Platelet Biology & its Disorders Hemoglobin interaction with GP1bα induces platelet activation and apoptosis: a novel mechanism associated with intravascular hemolysis Rashi Singhal,1,2,* Gowtham K. Annarapu,1,2,* Ankita Pandey,1 Sheetal Chawla,1 Amrita Ojha,1 Avinash Gupta,1 Miguel A. Cruz,3 Tulika Seth4 and Prasenjit Guchhait1 1Disease Biology Laboratory, Regional Centre for Biotechnology, National Capital Region, Biotech Science Cluster, Faridabad, India; 2Biotechnology Department, Manipal University, Manipal, Karnataka, India; 3Thrombosis Research Division, Baylor College of Medicine, Houston, TX, USA, and 4Hematology, All India Institute of Medical Sciences, New Delhi, India *RS and GKA contributed equally to this work. ABSTRACT Intravascular hemolysis increases the risk of hypercoagulation and thrombosis in hemolytic disorders. Our study shows a novel mechanism by which extracellular hemoglobin directly affects platelet activation. The binding of Hb to glycoprotein1bα activates platelets. Lower concentrations of Hb (0.37-3 mM) significantly increase the phos- phorylation of signaling adapter proteins, such as Lyn, PI3K, AKT, and ERK, and promote platelet aggregation in vitro. Higher concentrations of Hb (3-6 mM) activate the pro-apoptotic proteins Bak, Bax, cytochrome c, caspase-9 and caspase-3, and increase platelet clot formation. Increased plasma Hb activates platelets and promotes their apoptosis, and plays a crucial role in the pathogenesis of aggregation and development of the procoagulant state in hemolytic disorders. Furthermore, we show that in patients with paroxysmal nocturnal hemoglobinuria, a chronic hemolytic disease characterized by recurrent events of intravascular thrombosis and thromboembolism, it is the elevated plasma Hb or platelet surface bound Hb that positively correlates with platelet activation. -

Therapeutic Antibody-Like Immunoconjugates Against Tissue Factor with the Potential to Treat Angiogenesis-Dependent As Well As Macrophage-Associated Human Diseases
antibodies Review Therapeutic Antibody-Like Immunoconjugates against Tissue Factor with the Potential to Treat Angiogenesis-Dependent as Well as Macrophage-Associated Human Diseases Zhiwei Hu ID Department of Surgery Division of Surgical Oncology, The James Comprehensive Cancer Center, The Ohio State University College of Medicine, Columbus, OH 43210, USA; [email protected]; Tel.: +1-614-685-4606 Received: 10 October 2017; Accepted: 18 January 2018; Published: 23 January 2018 Abstract: Accumulating evidence suggests that tissue factor (TF) is selectively expressed in pathological angiogenesis-dependent as well as macrophage-associated human diseases. Pathological angiogenesis, the formation of neovasculature, is involved in many clinically significant human diseases, notably cancer, age-related macular degeneration (AMD), endometriosis and rheumatoid arthritis (RA). Macrophage is involved in the progression of a variety of human diseases, such as atherosclerosis and viral infections (human immunodeficiency virus, HIV and Ebola). It is well documented that TF is selectively expressed on angiogenic vascular endothelial cells (VECs) in these pathological angiogenesis-dependent human diseases and on disease-associated macrophages. Under physiology condition, TF is not expressed by quiescent VECs and monocytes but is solely restricted on some cells (such as pericytes) that are located outside of blood circulation and the inner layer of blood vessel walls. Here, we summarize TF expression on angiogenic VECs, macrophages and other diseased cell types in these human diseases. In cancer, for example, the cancer cells also overexpress TF in solid cancers and leukemia. Moreover, our group recently reported that TF is also expressed by cancer-initiating stem cells (CSCs) and can serve as a novel oncotarget for eradication of CSCs without drug resistance. -

Alternatively Spliced Tissue Factor Induces Angiogenesis Through Integrin Ligation
Alternatively spliced tissue factor induces angiogenesis through integrin ligation Y. W. van den Berga, L. G. van den Hengela, H. R. Myersa, O. Ayachia, E. Jordanovab, W. Rufc, C. A. Spekd, P. H. Reitsmaa, V. Y. Bogdanove, and H. H. Versteega,1 aThe Einthoven Laboratory for Experimental Vascular Medicine and bDepartment of Pathology, Leiden University Medical Center, Albinusdreef 2, 2333 ZA, Leiden, The Netherlands; cDepartment of Immunology and Microbial Science, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, CA 92037; dCenter for Experimental and Molecular Medicine, Academic Medical Center, Meibergdreef 9, 1105 AZ, Amsterdam, The Netherlands; and eDivision of Hematology/Oncology, Department of Internal Medicine, University of Cincinnati College of Medicine, 3125 Eden Avenue, Cincinnati, OH 45267 Edited by Charles T. Esmon, Oklahoma Medical Research Foundation, Oklahoma City, OK, and approved September 25, 2009 (received for review May 15, 2009) The initiator of coagulation, full-length tissue factor (flTF), in complex pancreatic cancer cells transfected to express asTF produce more with factor VIIa, influences angiogenesis through PAR-2. Recently, an blood vessels (20), but it remained mechanistically unclear if and alternatively spliced variant of TF (asTF) was discovered, in which part how angiogenesis is regulated by asTF. One possibility is that asTF of the TF extracellular domain, the transmembrane, and cytoplasmic stimulates cancer cells to produce angiogenic factors, but it is also domains are replaced by a unique C terminus. Subcutaneous tumors plausible that asTF enhances angiogenesis via paracrine stimulation produced by asTF-secreting cells revealed increased angiogenesis, but of endothelial cells. Moreover, the role of VIIa, PAR-2 activation it remained unclear if and how angiogenesis is regulated by asTF. -

Endotoxin Enhances Tissue Factor and Suppresses Thrombomodulin Expression of Human Vascular Endothelium in Vitro Kevin L
Endotoxin Enhances Tissue Factor and Suppresses Thrombomodulin Expression of Human Vascular Endothelium In Vitro Kevin L. Moore,* Sharon P. Andreoli,* Naomi L. Esmon,1 Charles T. Esmon,l and Nils U. Bang1l Department ofMedicine, Section ofHematology/Oncology,* and Department ofPediatrics, Section ofNephrology,t Indiana University School ofMedicine, Indianapolis, Indiana 46223; Oklahoma Medical Research Foundation,§ Oklahoma City, Oklahoma 73104; and Lilly Laboratory for Clinical Research,1' Indianapolis, Indiana 46202 Abstract to support the assembly of clotting factor complexes on their surfaces (13, 14). Endotoxemia is frequently associated clinically with disseminated Under physiologic conditions the thromboresistant properties intravascular coagulation (DIC); however, the mechanism of en- ofthe endothelium are predominant. In some pathological states, dotoxin action in vivo is unclear. Modulation of tissue factor however, this may not be the case. Gram-negative sepsis is fre- (TF) and thrombomodulin (TM) expression on the endothelial quently associated with varying degrees of disseminated intra- surface may be relevant pathophysiologic mechanisms. Stimu- vascular coagulation (DIC),' which is thought to be triggered by lation of human umbilical vein endothelial cells with endotoxin endotoxemia. The pathophysiology of DIC in gram-negative (1 ,ug/ml) increased surface TF activity from 1.52±0.84 to sepsis is complex and the mechanism(s) by which endotoxemia 11.89±8.12 mU/ml-106 cells at 6 h (n = 11) which returned to promotes intravascular coagulation in vivo is unclear. Recently, baseline by 24 h. Repeated stimulation at 24 h resulted in renewed reports by several investigators provide evidence that the throm- TF expression. Endotoxin (1 ,tg/ml) also caused a decrease in boresistance of the endothelial cell is diminished after exposure TM expression to 55.0±6.4% of control levels at 24 h (n = 10) to endotoxin in the absence of other cell types. -

A Differential Protein Solubility Approach for the Depletion of Highly Abundant Proteins in Plasma Using Ammonium Sulfate Ravi Chand Bollineni1, 2*, Ingrid J
Electronic Supplementary Material (ESI) for Analyst. This journal is © The Royal Society of Chemistry 2015 A differential protein solubility approach for the depletion of highly abundant proteins in plasma using ammonium sulfate Ravi Chand Bollineni1, 2*, Ingrid J. Guldvik3, Henrik Gronberg4, Fredrik Wiklund4, Ian G. Mills3, 5, 6 and Bernd Thiede1, 2 1Department of Biosciences, University of Oslo, Oslo, Norway 2Biotechnology Centre of Oslo, University of Oslo, Oslo, Norway 3Centre for Molecular Medicine Norway (NCMM), University of Oslo and Oslo University Hospitals, Norway 4Department of Medical Epidemiology and Biostatistics, Karolinska Institute, Stockholm, Sweden 5Department of Cancer Prevention, Oslo University Hospitals, Oslo, Norway 6Department of Urology, Oslo University Hospitals, Oslo, Norway Keywords: ammonium sulfate, blood, depletion, plasma, protein precipitation *To whom the correspondence should be addressed: Ravi Chand Bollineni, Department of Biosciences, University of Oslo, P.O. Box 1066 Blindern, 0316 Oslo, Norway, Tel.: +47-22840512; Fax +47-22840501; E-mail: [email protected] Supplementary information Figure S1: SDS-PAGE analysis of serum proteins precipitated with the ethanol/sodium acetate (A), TCA/acetone (B) and ammonium sulfate precipitation (C). A) Ethanol/sodium acetate precipitation: (1) pellet obtained after 42% ethanol precipitation and (2) pellet obtained after precipitation of proteins in the supernatant with 0.8M sodium acetate (pH5.7) and (3) proteins left over in the supernatant. B) Serum proteins are precipitated with 10% TCA/acetone (1) and (2) proteins left over in the supernatant. C) Serum proteins are precipitated with increasing ammonium sulfate concentrations 15% (1), 25% (2), 35% (3), 40% (4), 45% (5), 50% (6) and total serum (7). -

Supplementary Table S4. FGA Co-Expressed Gene List in LUAD
Supplementary Table S4. FGA co-expressed gene list in LUAD tumors Symbol R Locus Description FGG 0.919 4q28 fibrinogen gamma chain FGL1 0.635 8p22 fibrinogen-like 1 SLC7A2 0.536 8p22 solute carrier family 7 (cationic amino acid transporter, y+ system), member 2 DUSP4 0.521 8p12-p11 dual specificity phosphatase 4 HAL 0.51 12q22-q24.1histidine ammonia-lyase PDE4D 0.499 5q12 phosphodiesterase 4D, cAMP-specific FURIN 0.497 15q26.1 furin (paired basic amino acid cleaving enzyme) CPS1 0.49 2q35 carbamoyl-phosphate synthase 1, mitochondrial TESC 0.478 12q24.22 tescalcin INHA 0.465 2q35 inhibin, alpha S100P 0.461 4p16 S100 calcium binding protein P VPS37A 0.447 8p22 vacuolar protein sorting 37 homolog A (S. cerevisiae) SLC16A14 0.447 2q36.3 solute carrier family 16, member 14 PPARGC1A 0.443 4p15.1 peroxisome proliferator-activated receptor gamma, coactivator 1 alpha SIK1 0.435 21q22.3 salt-inducible kinase 1 IRS2 0.434 13q34 insulin receptor substrate 2 RND1 0.433 12q12 Rho family GTPase 1 HGD 0.433 3q13.33 homogentisate 1,2-dioxygenase PTP4A1 0.432 6q12 protein tyrosine phosphatase type IVA, member 1 C8orf4 0.428 8p11.2 chromosome 8 open reading frame 4 DDC 0.427 7p12.2 dopa decarboxylase (aromatic L-amino acid decarboxylase) TACC2 0.427 10q26 transforming, acidic coiled-coil containing protein 2 MUC13 0.422 3q21.2 mucin 13, cell surface associated C5 0.412 9q33-q34 complement component 5 NR4A2 0.412 2q22-q23 nuclear receptor subfamily 4, group A, member 2 EYS 0.411 6q12 eyes shut homolog (Drosophila) GPX2 0.406 14q24.1 glutathione peroxidase -

63Rd Annual SSC Meeting, in Conjunction with the 2017 ISTH Congress in Berlin, Germany Meeting Minutes
63rd Annual SSC meeting, in conjunction with the 2017 ISTH Congress in Berlin, Germany Meeting Minutes Standing Committees Coagulation Standards Committee ............................................................... 3 Subcommittees Animal, Cellular and Molecular Models ........................................................ 5 Biorheology .................................................................................................. 7 Control of Anticoagulation ............................................................................ 10 Disseminated Intravascular Coagulation ...................................................... 12 Factor VIII, Factor IX and Rare Coagulation Disorders ................................ 14 Factor XI and the Contact System ................................................................ 19 Factor XIII and Fibrinogen ............................................................................ 21 Fibrinolysis ................................................................................................... 27 Genomics in Thrombosis and Hemostasis ................................................... 33 Hemostasis and Malignancy......................................................................... 46 Lupus Anticoagulant/Phospholipid Dependent Antibodies ........................... 49 Pediatric and Neonatal Hemostasis and Thrombosis ................................... 54 Perioperative Thrombosis and Hemostasis .................................................. 59 Plasma Coagulation Inhibitors ..................................................................... -

Tissue Factor Regulation, Signaling and Functions Beyond Coagulation with a Focus on Diabetes
Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1626 Tissue Factor regulation, signaling and functions beyond coagulation with a focus on diabetes DESIRÉE EDÉN ACTA UNIVERSITATIS UPSALIENSIS ISSN 1651-6206 ISBN 978-91-513-0842-5 UPPSALA urn:nbn:se:uu:diva-399599 2020 Dissertation presented at Uppsala University to be publicly examined in Enghoffsalen, Akademiska sjukhuset, ing. 50, Uppsala, Friday, 21 February 2020 at 13:15 for the degree of Doctor of Philosophy (Faculty of Medicine). The examination will be conducted in Swedish. Faculty examiner: Docent, Universitetslektor Sofia Ramström (Örebro Universitet, Institutionen för medicinska vetenskaper). Abstract Edén, D. 2020. Tissue Factor regulation, signaling and functions beyond coagulation with a focus on diabetes. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Medicine 1626. 65 pp. Uppsala: Acta Universitatis Upsaliensis. ISBN 978-91-513-0842-5. Background: Tissue factor (TF) is a 47 kDa transmembrane glycoprotein best known for initiating the coagulation cascade upon binding of its ligand FVIIa. Apart from its physiological role in coagulation, TF and TF/FVIIa signaling has proved to be involved in diseases such as diabetes, cancer and cardiovascular diseases. Biological functions coupled to TF/FVIIa signaling include diet-induced obesity, apoptosis, angiogenesis and migration. Aim: The aim of this thesis was to investigate the role of TF/FVIIa in cells of importance in diabetes, to further investigate the mechanism behind TF/FVIIa anti-apoptotic signaling in cancer cells and lastly to examine the regulation of TF expression in monocytes by micro RNAs (miRNA). Results: In paper I we found that TF/FVIIa signaling augments cytokine-induced beta cell death and impairs glucose stimulated insulin secretion from human pancreatic islets. -

General Considerations of Coagulation Proteins
ANNALS OF CLINICAL AND LABORATORY SCIENCE, Vol. 8, No. 2 Copyright © 1978, Institute for Clinical Science General Considerations of Coagulation Proteins DAVID GREEN, M.D., Ph.D.* Atherosclerosis Program, Rehabilitation Institute of Chicago, Section of Hematology, Department of Medicine, and Northwestern University Medical School, Chicago, IL 60611. ABSTRACT The coagulation system is part of the continuum of host response to injury and is thus intimately involved with the kinin, complement and fibrinolytic systems. In fact, as these multiple interrelationships have un folded, it has become difficult to define components as belonging to just one system. With this limitation in mind, an attempt has been made to present the biochemistry and physiology of those factors which appear to have a dominant role in the coagulation system. Coagulation proteins in general are single chain glycoprotein molecules. The reactions which lead to their activation are usually dependent on the presence of an appropriate surface, which often is a phospholipid micelle. Large molecular weight cofactors are bound to the surface, frequently by calcium, and act to induce a favorable conformational change in the reacting molecules. These mole cules are typically serine proteases which remove small peptides from the clotting factors, converting the single chain species to two chain molecules with active site exposed. The sequence of activation is defined by the enzymes and substrates involved and eventuates in fibrin formation. Mul tiple alternative pathways and control mechanisms exist throughout the normal sequence to limit coagulation to the area of injury and to prevent interference with the systemic circulation. Introduction RatnofP4 eloquently indicates in an arti cle aptly entitled: “A Tangled Web. -
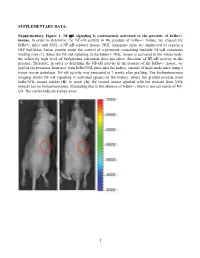
Activation of NF-Κb Signaling Promotes Prostate Cancer Progression in the Mouse and Predicts Poor Progression and Death in Pati
SUPPLEMENTARY DATA: Supplementary Figure 1. NF-B signaling is continuously activated in the prostate of - mouse. In order to determine the NF- '- mouse, we crossed the '- mice with NGL, a NF-B reporter mouse. NGL transgenic mice are engineered to express a GFP/luciferase fusion protein under the control of a promoter containing multiple NF-B consensus binding sites (1). Since the NF-'- NGL mouse is activated in the whole body, the relatively high level of background activation does not allow detection of NF- prostate. Therefore, in order to determine the NF-the '- mouse, we grafted the prostates from 'o the kidney capsule of male nude mice using a tissue rescue technique. NF-B activity was measured at 7 weeks after grafting. The bioluminescence imaging shows NF-B signaling is activated (green) in the kidney, where the grafted prostate from ' use resides (B). In panel (A), the control mouse (grafted with the prostate from NGL mouse) has no bioluminescence, illustrating that in the absence of '-, there is not activation of NF- B. The circles indicate kidney areas. 1 Supplementary Figure 2. NF-B signaling activated in the prostate of Myc/IB bigenic mouse. The prostates from Myc alone (Myc) and bigenic (Myc/IB) mice were harvested at 6 months of age. Activation of NF-B signaling in the prostate was determined by IHC staining of p65-pho antibody. 2 Supplementary Figure 3. Continuous activation of NF-B signaling promotes PCa progression in the Hi-Myc transgenic mouse. The prostates from Myc alone (Myc) and bigeneic (Myc/IB) mice were harvested at 6 months of age. -

Thromboplastin, Tissue Factor
TNF_repressed genes, fat tissue, 1 day infusion Description Accession Fold Blood Coagulation Coagulation factor III (thromboplastin, tissue factor) U07619 1.4 factor XIIIa Y12502 1.5 Cell Adhesion integrin alpha chain, H36-alpha7 X65036 1.6 integrin alpha-1 X52140 1.4 Cell Cycle Control-Cell Stress growth arrest and DNA-damage-inducible (GADD45) L32591 1.5 Cell Signaling adipocyte hormone-sensitive cyclic AMP phosphodiesterase Z22867 1.5 Fibroblast growth factor receptor 1 beta-isoform S54008 1.4 Insulin-like growth factor binding protein 6 M69055 1.9 LIM domain kinase 1 isoform 1 (LIMK-1) D31873 1.4 protein kinase PASK D88190 2.2 protein phosphatase 1 beta S78218 1.4 protein phosphatase inhibitor-1 protein J05592 1.9 S-100 beta subunit S53527 1.6 Cell Stress Glutathione S-transferase 1 (theta) D10026 1.6 heat shock related protein AI171166 1.5 selenoprotein P AI230247 1.4 Cell Structure and Cytoskeleton myosin regulatory light chain isoform C S77900 1.7 Cytokine cardiotrophin-1 D78591 1.5 Transforming growth factor, beta 3 U03491 1.8 DNA Synthesis nuclear factor I/A (NFI-A1) X84210 1.4 nuclear factor I/B (NF1-B2) AB012231 1.6 nuclear factor I/B (NF1-B3) AI176488 1.6 Immune Response mast cell protease 1 precursor (RMCP-1) U67915 1.5 MHC class II A-beta RT1.B-b-beta gene M36151 2.2 MHC class II antigen RT1.B-1 beta-chain X56596 1.8 MHC class II RT1.B-alpha chain gene X07551 2.2 MHC class II RT1.u-D-alpha chain mRNA M15562 2 MHC class II-associated invariant chain X14254 2.2 MHC RT1-B region class II (Ia antigen) K02815 1.8 MHC-associated -

Carboxypeptidase B2 (CPB2) Human Shrna Lentiviral Particle (Locus ID 1361) Product Data
OriGene Technologies, Inc. 9620 Medical Center Drive, Ste 200 Rockville, MD 20850, US Phone: +1-888-267-4436 [email protected] EU: [email protected] CN: [email protected] Product datasheet for TL305252V Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Product data: Product Type: shRNA Lentiviral Particles Product Name: Carboxypeptidase B2 (CPB2) Human shRNA Lentiviral Particle (Locus ID 1361) Locus ID: 1361 Synonyms: CPU; PCPB; TAFI Vector: pGFP-C-shLenti (TR30023) Format: Lentiviral particles RefSeq: NM_001278541, NM_001872, NM_016413, NM_001872.1, NM_001872.2, NM_001872.3, NM_001872.4, NM_016413.1, NM_016413.2, NM_016413.3, NM_001278541.1, BC007057, BC007057.1, NM_001872.5 Summary: Carboxypeptidases are enzymes that hydrolyze C-terminal peptide bonds. The carboxypeptidase family includes metallo-, serine, and cysteine carboxypeptidases. According to their substrate specificity, these enzymes are referred to as carboxypeptidase A (cleaving aliphatic residues) or carboxypeptidase B (cleaving basic amino residues). The protein encoded by this gene is activated by trypsin and acts on carboxypeptidase B substrates. After thrombin activation, the mature protein downregulates fibrinolysis. Polymorphisms have been described for this gene and its promoter region. Alternate splicing results in multiple transcript variants. [provided by RefSeq, Jun 2013] shRNA Design: These shRNA constructs were designed against multiple splice variants at this gene locus. To be certain that your variant of interest is targeted,