Isothermal and Non-Isothermal Kinetic and Safety Parameter Evaluation of Tert-Butyl(2-Ethylhexyl)Monoperoxy Carbonate by Differential Scanning Calorimetry
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Achievements of MCU Representative Sport Teams at 2016-17 University Championship Competitions
Achievements of MCU Representative Sport Teams at 2016-17 University Championship Competitions Serial Participant Name of Competition Date & Venue Place No. s 2016-17 Taiwan University December 7-10, 2016 First Place in 2 1 Petanque Championship TianMu Petanque Field Mixed Teams 2016-17 Taiwan University December 7-10, 2016 First Place in 3 2 Petanque Championship TianMu Petanque Field Women’s Triples 2016-17 Taiwan University December 7-10, 2016 First Place in 2 3 Petanque Championship TianMu Petanque Field Women’s Doubles 2016-17 Taiwan University December 7-10, 2016 First Place in 2 4 Petanque Championship TianMu Petanque Field Women’s Doubles 2016-17 Taiwan University December 7-10, 2016 First Place in 1 5 Petanque Championship TianMu Petanque Field Women’s Triples 2017 Taiwan University First Place in Women’s Team 4 May 7-10, 2017 6 Sports Festival- Fencing Epee National Taiwan University Championship 2017 Taiwan University First Place in Women’s 1 May 7-10, 2017 7 Sports Festival- Fencing Individual Epee National Taiwan University Championship 2017 Taiwan University May 6-10, 2017 First Place in Women’s 1 8 Sports Festival- Woodball National Taipei University of Nursing Individual Stroke Championship and Health Sciences 2017 Taiwan University May 6-10, 2017 First Place in Women’s 1 9 Sports Festival- Woodball National Taipei University of Nursing Individual Fairway Championship and Health Sciences 2016 National Chung-Cheng First Place in University 16 Cup University and Oct. 15-26, 2016 Women’s A Level 2 10 Secondary School Miaoli County Stadium Basketball Championship “Cheer for Summer First Place in Dancing 9 Dec. -
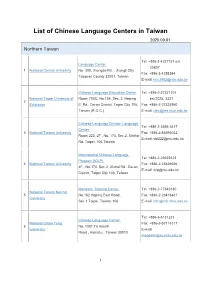
List of Chinese Language Centers in Taiwan 2020.09.01
List of Chinese Language Centers in Taiwan 2020.09.01 Northern Taiwan Tel: +886-3-4227151 ext. Language Center 33807 1 National Central University No. 300, Jhongda Rd. , Jhongli City , Fax: +886-3-4255384 Taoyuan County 32001, Taiwan E-mail: [email protected] Chinese Language Education Center Tel: +886-2-27321104 National Taipei University of Room 700C, No.134, Sec. 2, Heping ext.2025, 3331 2 Education E. Rd., Da-an District, Taipei City 106, Fax: +886-2-27325950 Taiwan (R.O.C.) E-mail: [email protected] Chinese Language Division Language Tel: +886-2-3366-3417 Center 3 National Taiwan University Fax: +886-2-83695042 Room 222, 2F , No. 170, Sec.2, XinHai E-mail: [email protected] Rd, Taipei, 106,Taiwan International Chinese Language Tel: +886-2-23639123 Program (ICLP) 4 National Taiwan University Fax: +886-2-23626926 4F., No.170, Sec.2, Xinhai Rd., Da-an E-mail: [email protected] District, Taipei City 106, Taiwan Mandarin Training Center Tel: +886-2-77345130 National Taiwan Normal 5 No.162 Hoping East Road , Fax: +886-2-23418431 University Sec.1 Taipei, Taiwan 106 E-mail: [email protected] Tel: +886-3-5131231 Chinese Language Center National Chiao Tung Fax: +886-3-57114317 6 No. 1001 Ta Hsueh University E-mail: Road , Hsinchu , Taiwan 30010 [email protected] 1 Chinese Language Center Tel: +886-2-2938-7141/7142 No.64, Sec. 2, Zhinan Rd., Wenshan Fax: +886-2-2939-6353 7 National Chengchi University District E-mail: Taipei City 11605, Taiwan (R.O.C.) [email protected] Tel: +886-2-2700-5858 Mandarin Learning Center ext.8131~8136 8 Chinese Culture University 4F , No.231, Sec.2, Chien-Kuo S. -

A Reflective Self-Inquiry Into My Teaching Practices with Non-English-Major Technological University Students
Canadian Journal of Action Research Volume 14, Issue 3, 2013, pages 59-71 A REFLECTIVE SELF-INQUIRY INTO MY TEACHING PRACTICES WITH NON-ENGLISH-MAJOR TECHNOLOGICAL UNIVERSITY STUDENTS Chiu-Kuei Chang Chien, WuFeng University, Taiwan Kuo-Jen Yu, Nanhua University, Taiwan Lung-Chi Lin, Kao Yuan University, Taiwan ABSTRACT Action research allows teachers to evaluate and gain insight into their own practices in their teaching contexts through reflection, and inquire into ways to improve their practices and student learning outcomes. This study is a reflective self-inquiry in which the research team engages in a deliberate and retrospective analysis of the principal investigator’s teaching practices. The purpose is to identify possible factors that cause the low performance of non- major “English as a Foreign Language” students in her university English classes and propose alternative instructional practices and evaluative measures to manage their learning problems. INTRODUCTION Action research allows teachers to gain insight into their own practices in their teaching contexts through reflection and evaluation, and to inquire into methods that can help improve student practices and learning outcomes. It comprises a broad spectrum of approaches to action, change, and inquiries. One form of action research is self-reflective inquiry by which practitioners seek to solve problems, improve practices, and enhance their understanding of their own contexts (Kemmis & McTaggart, 1988; Nunan, 1992). Inquiry is intrinsically a behavior or course of finding -

A Study on the Development of Advanced Faculty in Leisure and Sport for Higher Education Between 2006 and 2011
A Study on the Development of Advanced Faculty in Leisure and Sport for Higher Education between 2006 and 2011 Cheng-Lung Wu, Department of Marine Sports and Recreation, National Penghu University of Science and Technology, Taiwan Yung-Chuan Tsai, Department of Recreation Sports & Health Promotion, Meiho University, Taiwan Yong Tang, Corresponding Author, Department of Aquatic Sport and Recreation, Taipei College of Maritime Technology, Taiwan ABSTRACT This study employed latent growth curve modeling to verify the growth on the number of full-time faculty in the department relevant to leisure and sports in higher educational institutions in Taiwan. The scope of this study covers the departments relevant to leisure and sport. The number of the full-time faculty in the department relevant to leisure and sport was treated as observed variable for the analysis of its growth. The results found that a goodness fit between the number of students recruited in the latent growth curve model and observed data and that the growth of the number of the faculty in the department relevant to leisure and sport was significantly different along with time and the increase of the number of classes in the department relevant to the leisure and sport. And, the number of the faculty in the department relevant to leisure and sport in between 2006 and 2011 was significantly impacted by the higher educational systems, traditional or vocational universities, both of the number in the beginning point and growth rate were significantly different. It revealed that the factor of traditional and vocational educational system did influence the number of full-time faculty. -

BAI / ISTEL Tentative Conference Program
BAI / ISTEL Tentative Conference Program Tuesday, July 04, 2017 Time 14:00-16:00 Registration 14:30-16:00 Opening Ceremony Host: Ushio Sumita, Keio University, Japan Keynote Speech Fernando A. F. Ferreira, ISCTE University Institute of Lisbon, Portugal Distinguished Paper Awards Wednesday, July 05, 2017 Time 09:00-17:00 Registration 09:15-10:30 A1 B1 C1 D1 E1 10:30-10:45 Poster Session 1 & Coffee Break 10:45-12:00 A2 B2 C2 D2 E2 12:00-13:30 Lunch 13:30-14:45 A3 B3 C3 D3 E3 14:45-15:00 Poster Session 2 & Coffee Break 15:00-16:15 A4 B4 C4 D4 E4 16:15-16:30 Poster Session 3 & Coffee Break 16:30-17:45 A5 B5 C5 D5 E5 Thursday, July 06, 2017 Time 09:00-15:00 Registration 09:15-10:30 A6 B6 C6 D6 E6 10:30-10:45 Poster Session 4 & Coffee Break 10:45-12:00 A7 B7 C7 D7 E7 12:00-13:30 Lunch 13:30-14:45 A8 B8 C8 D8 E8 14:45-15:00 Poster Session 5 & Coffee Break 15:00-16:15 A9 B9 C9 D9 ────────────────────────────────────────── 09:15-10:30 | Wednesday, July 05, 2017 Session: A1 ────────────────────────────────────────── A Study of Buyer’s Dependence Antecedents Feng Hsu Liu, Shih Hsin University Japan’s Outbound Tourism Trends: Managing Multiple Source Markets in Asia Pacific Destinations Fred Robert Schumann, University of Guam Demographic Analysis for the Social Entrepreneurial Intentions of Media Workers Huei-Ching Liu, TVBS TV Network Ching Yin Ip, National Taiwan University Chaoyun Liang, National Taiwan University New Explanation on Bertrand Paradox and Price War Zijian Jin, Wuyi University Weilong Yang, Wuyi University ────────────────────────────────────────── -

Student Version Academic and Internship Handbook For
Academic and Internship Handbook for International and Overseas Chinese Students-Student Version 52 Preface Welcome to Taiwan, the Republic of China! Taiwan is blessed with beautiful scenery, a pleasant climate and earnest local people. Our campus has a lively atmosphere, with caring teachers and helpful students. Studying here, not only can you acquire knowledge Welcome to Taiwan ! and expertise in the classroom and participate in diverse extracurricular activities in school, you can also explore the country more thoroughly in your free time, learning Taiwanese culture, tasting local delicacies and visiting famous attractions. On your arrival, you will definitely be thrilled by what you see; the next few years of studying here will, I am sure, leave an unforgettable, beautiful memory in your life. However, local customs, laws and regulations in Taiwan are different from other During your study in Taiwan, in addition to scheduling classroom courses, your countries. To equip you with guidance on schooling and living so that you won’t be at a academic department may arrange internship programs according to relevant regulations, loss in times of trouble, this reference manual has been purposely put together to provide provided they are part of your study, so that you can learn the nature and requirements of information on the problems you may encounter in your studies, internship and daily life, the workplace in your field of study, as well as enabling mutual corroboration of theory as well as their solutions. The information in this manual is for reference only; for matters and practice. Please be aware that the regulations on internship and working part-time not mentioned herein, please consult the designated office in your school. -

The Effect of Tertiary Education Expansion on Fertility: a Note on Identification
DISCUSSION PAPER SERIES IZA DP No. 14672 ‘Bachelors’ and Babies: The Effect of Tertiary Education Expansion on Fertility Tushar Bharati Simon Chang Qing Li AUGUST 2021 DISCUSSION PAPER SERIES IZA DP No. 14672 ‘Bachelors’ and Babies: The Effect of Tertiary Education Expansion on Fertility Tushar Bharati Qing Li University of Western Australia Business Shanghai University School Simon Chang University of Western Australia Business School and IZA AUGUST 2021 Any opinions expressed in this paper are those of the author(s) and not those of IZA. Research published in this series may include views on policy, but IZA takes no institutional policy positions. The IZA research network is committed to the IZA Guiding Principles of Research Integrity. The IZA Institute of Labor Economics is an independent economic research institute that conducts research in labor economics and offers evidence-based policy advice on labor market issues. Supported by the Deutsche Post Foundation, IZA runs the world’s largest network of economists, whose research aims to provide answers to the global labor market challenges of our time. Our key objective is to build bridges between academic research, policymakers and society. IZA Discussion Papers often represent preliminary work and are circulated to encourage discussion. Citation of such a paper should account for its provisional character. A revised version may be available directly from the author. ISSN: 2365-9793 IZA – Institute of Labor Economics Schaumburg-Lippe-Straße 5–9 Phone: +49-228-3894-0 53113 Bonn, Germany -

Taiwan's Education and Scholarships for Cultivating Talents for the 10
Taiwan’s Education and Scholarships for Cultivating Talents for the 10 Targeted Industries in the “Thailand 4.0” Scheme Compiled by Taipei Economic & Cultural Office in Thailand Published on 9 October, 2019 Taiwan’s Education and Scholarships for Cultivating Talents for the 10 Targeted Industries in the “Thailand 4.0” Scheme Thailand 4.0 is a new model being proposed by the Thai government as they look towards having a value-based economy. There are 10 targeted industries in Thailand 4.0 scheme, including Next-Generation Automotive, Smart Electronics, Affluent, Medical and Wellness Tourism, Agriculture and Biotechnology, Food for the Future, Robotics, Medical Hub, Aviation and Logistics, Biofuels and Biochemicals, and Digital Economy. The compiled list of departments and graduate institutes from Academia Sinica and 32 Taiwanese universities below is about Taiwan’s education for cultivating Thai talents for the 10 targeted industries in the “Thailand 4.0” scheme. In order to support Thai students to pursue their advanced studies in Taiwan, there are approximately 1,200 scholarships (including university scholarships and government scholarships) qualified for Thai students to apply. Not only the Academia Sinica and 32 Taiwanese prestigious universities provide their own scholarships (please check the Admission offices links below), but also Taiwanese government supports three scholarships, including “The Ministry of Education (MOE) Taiwan Scholarship Program”, “The Ministry of Science and Technology (MOST) Taiwan Scholarship Program”, and “The TaiwanICDF International Higher Education Scholarship Program.” In particular, the “MOE”, “MOST”, and “TaiwanICDF” three Taiwanese government scholarship programs provide around 20-25 full scholarships for Thai students to pursue their undergraduate and graduate degrees in Taiwan, including tuitions, airfares, and monthly living allowance between 1 NT$12,000 to NT$30,000. -

Mechanical and Microstructural Characterization of Alkali-Activated Materials Based on Fly Ash and Slag
IACSIT International Journal of Engineering and Technology, Vol. 7, No. 1, February 2015 Mechanical and Microstructural Characterization of Alkali-Activated Materials Based on Fly Ash and Slag Maochieh Chi, Yenchun Liu, and Ran Huang strength have been obtained in alkali-activated GGBFS Abstract—This study investigates the mechanical and mortars or concretes than comparable OPC mortars or microstructural characterization of alkali-activated fly ash/slag concretes [11]. However, its drying shrinkage of (AAFS) mortars with various ratios of fly ash to slag. The alkali-activated slag concrete is higher than that of OPC liquid/binder ratios of 0.35, 0.5 and 0.65 are considered for the concrete [12]. In contrast, fly ash based alkali-activated AAFS mortars. 4% Sodium oxide (Na2O) concentration of cementitious material weight in mixture and liquid sodium mortars or concretes usually exhibit a slower setting and silicate with modulus ratio (mass ratio of SiO2 to Na2O) of 1 strength development [13]. The key factors influencing their were used as alkaline activators to alkali-activate various fly potential reactivity are the reactive silica content, the vitreous ash/slag ratios. Compressive strength test, water absorption test, phase content and the particle size distribution [2]. Palomo et drying shrinkage test, scanning electron microscopy (SEM) and al. [14] reported that temperature, curing time of specimens X-ray diffraction (XRD) analysis were conducted. Test results and solution/fly ash ratio have a significant influence on the reveal that both fly ash/slag ratio and the liquid/binder ratio are two significant factors influencing the mechanical and mechanical strength of the alkali-activated fly ash pastes. -

List of Reviewers 2018
78 List of Reviewers 2018 We gratefully acknowledge the contribution of the following reviewers who reviewed papers for Research in Journal of Marine Science and Technology in 2018. Alberto Magreñán, University of La Rioja, Spain Apostolos D. Papanikolaou, National Technical University of Athens, Greece Bao-Shi Shiau, National Taiwan Ocean University, Taiwan (R.O.C.) Beom-Seon Jang, Seoul National University, Korea Bin Ji, Huazhong University Science and Technology, China Bo Zhou, Nanyang Technological University, Singapore Chen-Far Hung, National Taiwan University, Taiwan (R.O.C.) Cheng-Chi Chung, National Taiwan Ocean University, Taiwan (R.O.C.) Cheng-Wen Lin, China Corporation Register of Shipping (CR), Taiwan (R.O.C.) Cheng-Yi Chen, Cheng Shiu University, Taiwan (R.O.C.) Cheng-Yu Ku, National Taiwan Ocean University, Taiwan (R.O.C.) Chen-Huei Huang, National Chiayi University, Taiwan (R.O.C.) Chen-Ming Chu, National Iland Univerisity, Taiwan (R.O.C.) Cheung-Chieh Ku, National Taiwan Ocean University, Taiwan (R.O.C.) Chia Hao Chang, Tonghai University, Taiwan (R.O.C.) Chia-Chi Sung, National Taiwan University, Taiwan (R.O.C.) Chia-Ming Fan, National Taiwan Ocean University, Taiwan (R.O.C.) Chien-Chang Chou, National Kaohsiung University of Science and Technology, Taiwan (R.O.C.) Chien-Kuei Yang, Ming Chuan University, Taiwan (R.O.C.) Chih-Min Hsieh, National Kaohsiung University of Science and Technology, Taiwan (R.O.C.) Chih-Wei Yen, Industrial Technology Research Institute of Taiwan, R.O.C, Taiwan (R.O.C.) Ching-Hsiang Tseng, National -

Download Article
International Conference on Education, Management, Computer and Society (EMCS 2016) Corporations Development Stage and Relationship MIS and TQM in the E- Business Era Chiachia Lin Wentan Wu* Department of Tourism and Leisure Management Institute of Leisure Recreation and Travel Management WuFeng University ToKo University Chia-yi, Taiwan Chia- yi, Taiwan [email protected]; 46cclin@ gmail.com [email protected] * Corresponding Author Hungyuan Huang Huanming Chuang Institute of Business Administration Department of Information Management Nanhua University National Yunlin University of Science and Technology Chia-yi, Taiwan Yun-lin, Taiwan [email protected] [email protected] Abstract—This paper intends to investigate how implementation of management information system, corporations introduce and develop management enterprises should be able to better execute total quality information system (MIS) and total quality management management. (TQM) in facing the e-commerce era. Several 2014 national Taiwan National Quality Award (TNQA) is the top quality award winning firms will be used as individual cases honor awarding to enterprises with overall business quality for in-depth analysis. The development of a company’s MIS in Taiwan. In 1990, The Ministry of Economic Affairs and TQM will be analyzed. In this study, we discover the established the TNQA to help enterprises upgrade overall development of MIS and TQM for a company is closely business standard and improve international image. related. Those award winning firms maintained a very high Initially the award was limited to manufacturers only in standard in the development of both MIS and TQM. This manufacturing industry, starting in 1995 the award was study used evaluation criteria of the national quality award as the foundation to discuss the correlation and influence of applicable to non-manufacturing industry. -

Enriched Player Situated Learning in ... -.:: Natural Sciences Publishing
Appl. Math. Inf. Sci. 7 No. 1S pp. 309S-317S (2013) Applied Mathematics & Information Sciences An International Journal © 2012 NSP @ 2012 NSP Natural Sciences Publishing Cor. Enriched Player Situated Learning in Serious Game for Cultural Heritage Learning Jung -Tsung Chen1, Chih Ming Chiu2, Shao-Shin Hung3, Derchian Tsaih4, Hui-Ling Lin5,6 and Jyh-Jong Tsay7 1 Department of Applied Game Technology, WuFeng University, Chiayi, Taiwan 2 Department of Computer Science and Information Engineering, National Chung Cheng University, Chiayi, Taiwan 3 Department of Computer Science and Information Engineering, WuFeng University, Chiayi, Taiwan 4 Department of Electronic Commerce Management, Nanhua University, Chiayi, Taiwan 5 Department of Industrial Engineering and Enterprise Information, Tunghai University, Taichung, Taiwan 6 Department of International Business, Ling Tung University, Taichung, Taiwan 7 Department of Computer Science and Information Engineering, National Chung Cheng University, Chiayi, Taiwan Corresponding author: Email: [email protected] Received Nov. 25, 2011; Revised Jan. 8, 2012; Accepted Jan. 31, 2012 Published online: 2012 Abstract: Living and being part of today’s Knowledge Society implies recognizing the importance of the past and imposes considering cultural heritage as a fundamental background of our identity. This paper investigates how a serious game can contribute to enhancing cultural heritage education. It is an attempt to answer the question concerning whether serious game can really provide any added value to cultural heritage pedagogy, education and learning. By focusing on those cultural heritage artifacts that pertain to the field of arts and archaeology, the paper assumes a methodological perspective and provides examples of some of the most innovative experiences in the field, thus driving the reader to reflect on the pedagogical impact that may derive from exploiting serious game potentialities.