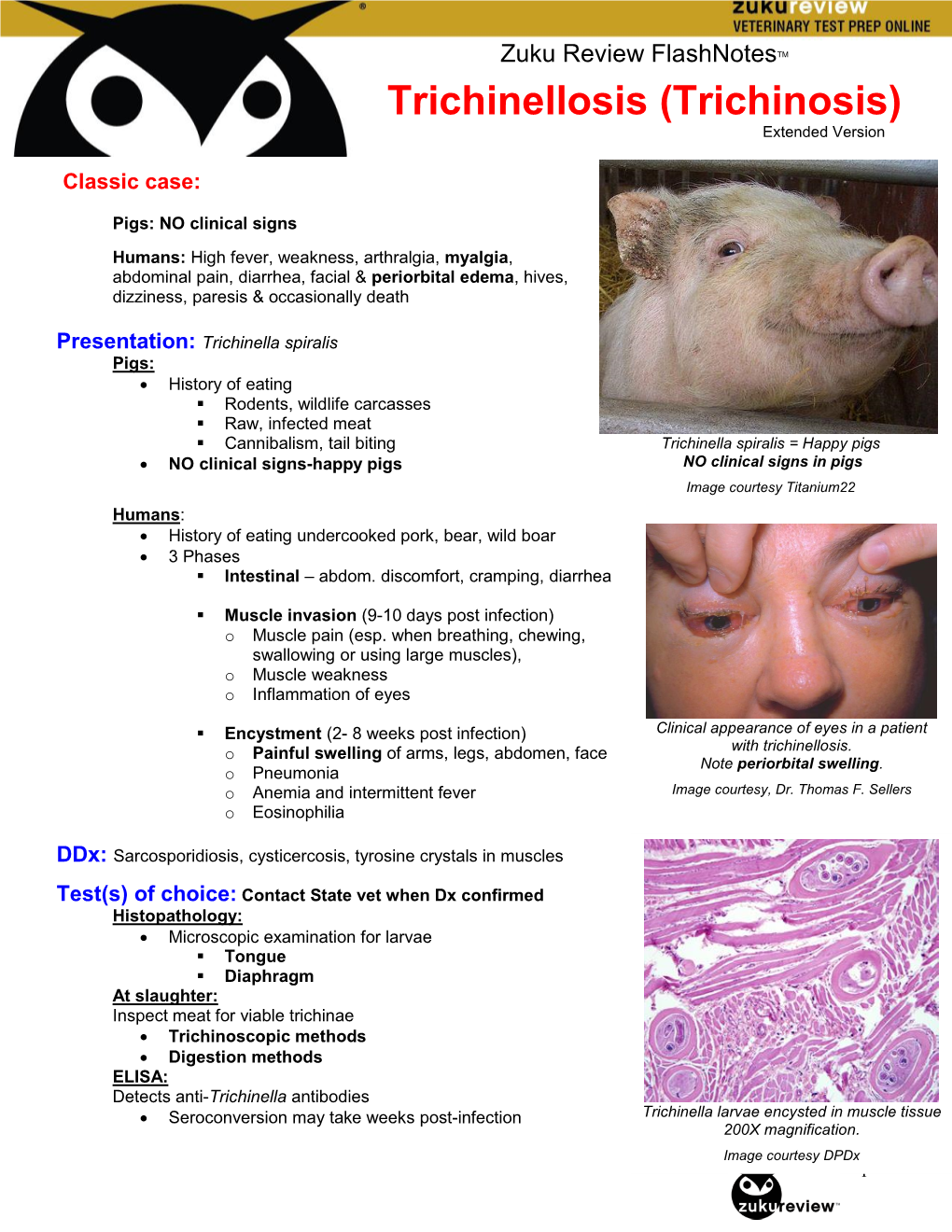
Trichinellosis (Trichinosis) Extended Version
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

CDC Overseas Parasite Guidelines
Guidelines for Overseas Presumptive Treatment of Strongyloidiasis, Schistosomiasis, and Soil-Transmitted Helminth Infections for Refugees Resettling to the United States U.S. Department of Health and Human Services Centers for Disease Control and Prevention National Center for Emerging and Zoonotic Infectious Diseases Division of Global Migration and Quarantine February 6, 2019 Accessible version: https://www.cdc.gov/immigrantrefugeehealth/guidelines/overseas/intestinal- parasites-overseas.html 1 Guidelines for Overseas Presumptive Treatment of Strongyloidiasis, Schistosomiasis, and Soil-Transmitted Helminth Infections for Refugees Resettling to the United States UPDATES--the following are content updates from the previous version of the overseas guidance, which was posted in 2008 • Latin American and Caribbean refugees are now included, in addition to Asian, Middle Eastern, and African refugees. • Recommendations for management of Strongyloides in refugees from Loa loa endemic areas emphasize a screen-and-treat approach and de-emphasize a presumptive high-dose albendazole approach. • Presumptive use of albendazole during any trimester of pregnancy is no longer recommended. • Links to a new table for the Treatment Schedules for Presumptive Parasitic Infections for U.S.-Bound Refugees, administered by IOM. Contents • Summary of Recommendations • Background • Recommendations for overseas presumptive treatment of intestinal parasites o Refugees originating from the Middle East, Asia, North Africa, Latin America, and the Caribbean o Refugees -

Angiostrongylus Cantonensis: a Review of Its Distribution, Molecular Biology and Clinical Significance As a Human
See discussions, stats, and author profiles for this publication at: https://www.researchgate.net/publication/303551798 Angiostrongylus cantonensis: A review of its distribution, molecular biology and clinical significance as a human... Article in Parasitology · May 2016 DOI: 10.1017/S0031182016000652 CITATIONS READS 4 360 10 authors, including: Indy Sandaradura Richard Malik Centre for Infectious Diseases and Microbiolo… University of Sydney 10 PUBLICATIONS 27 CITATIONS 522 PUBLICATIONS 6,546 CITATIONS SEE PROFILE SEE PROFILE Derek Spielman Rogan Lee University of Sydney The New South Wales Department of Health 34 PUBLICATIONS 892 CITATIONS 60 PUBLICATIONS 669 CITATIONS SEE PROFILE SEE PROFILE Some of the authors of this publication are also working on these related projects: Create new project "The protective rate of the feline immunodeficiency virus vaccine: An Australian field study" View project Comparison of three feline leukaemia virus (FeLV) point-of-care antigen test kits using blood and saliva View project All content following this page was uploaded by Indy Sandaradura on 30 May 2016. The user has requested enhancement of the downloaded file. All in-text references underlined in blue are added to the original document and are linked to publications on ResearchGate, letting you access and read them immediately. 1 Angiostrongylus cantonensis: a review of its distribution, molecular biology and clinical significance as a human pathogen JOEL BARRATT1,2*†, DOUGLAS CHAN1,2,3†, INDY SANDARADURA3,4, RICHARD MALIK5, DEREK SPIELMAN6,ROGANLEE7, DEBORAH MARRIOTT3, JOHN HARKNESS3, JOHN ELLIS2 and DAMIEN STARK3 1 i3 Institute, University of Technology Sydney, Ultimo, NSW, Australia 2 School of Life Sciences, University of Technology Sydney, Ultimo, NSW, Australia 3 Department of Microbiology, SydPath, St. -

Tissue Nematode: Trichenella Spiralis
Tissue nematode: Trichenella spiralis Introduction Trichinella spiralis is a viviparous nematode parasite, occurring in pigs, rodents, bears, hyenas and humans, and is liable for the disease trichinosis. It is occasionally referred to as the "pork worm" as it is characteristically encountered in undercooked pork foodstuffs. It should not be perplexed with the distantly related pork tapeworm. Trichinella species, the small nematode parasite of individuals, have an abnormal lifecycle, and are one of the most extensive and clinically significant parasites in the whole world. The adult worms attain maturity in the small intestine of a definitive host, such as pig. Each adult female gives rise to batches of live larvae, which bore across the intestinal wall, enter the blood stream (to feed on it) and lymphatic system, and are passed to striated muscle. Once reaching to the muscle, they encyst, or become enclosed in a capsule. Humans can become infected by eating contaminated pork, horse meat, or wild carnivorus animals such as cat, fox, or bear. Epidemology Trichinosis is a disease caused by the worm. It occurs around most parts of the world, and infects majority of humans. It ranges from North America and Europe, to Japan, China and Tropical Africa. Morphology Males of T. spiralis measure about1.4 and 1.6 mm long, and are more flat at anterior end than posterior end. The anus is seen in the terminal position, and they have a large copulatory pseudobursa on both the side. The females of T. spiralis are nearly twice the size of the males, and have an anal aperture situated terminally. -

Onchocerciasis, Cysticercosis, and Epilepsy
Am. J. Trop. Med. Hyg., 79(5), 2008, pp. 643–645 Copyright © 2008 by The American Society of Tropical Medicine and Hygiene Letter to the Editor Onchocerciasis, Cysticercosis, and Epilepsy Dear Sir: detected cysticercal antibodies in 17 (18.3%) of 93 patients with epilepsy compared with 12 (14.8%) of 81 controls (a confidence 95% ,1.3 ס Katabarwa and others1 reported on detection of nodules of non-significant difference; odds ratio This finding suggests that in the areas of 9.(3.0–0.6 ס Taenia solium cysticerci mistakenly identified as Onchocerca interval volvulus nodules in a post-treatment survey after 12 years of the studies conducted in Burundi3 and Cameroon,4 where O. ivermectin mass treatment in Uganda. On the basis of this volvulus and T. solium are co-endemic, the expected influ- observation, they suggest that neurocysticercosis may be the ence of cysticercosis on epilepsy may be masked by the effect cause of frequent occurrence of epileptic seizures, which has of onchocerciasis as a competing etiologic factor. been reported from several onchocerciasis-endemic areas. As demonstrated by Katabarwa and others1 and in other Over the past 15 years, a positive correlation between the studies, T. solium infection is widespread in Africa and can be prevalence of epilepsy and that of onchocerciasis has been co-endemic with onchocerciasis in many areas. We agree with reported from various African areas.2–5 All of these studies Katabarwa and others1 that subcutaneous cysticercal cysts used microscopy for the detection of microfilariae in dermal may be confounded with onchocercal nodules in co-endemic biopsy specimens as an unequivocal diagnostic means of in- areas, and that this is of relevance because it could produce a fection with O. -

Clinical Cysticercosis: Diagnosis and Treatment 11 2
WHO/FAO/OIE Guidelines for the surveillance, prevention and control of taeniosis/cysticercosis Editor: K.D. Murrell Associate Editors: P. Dorny A. Flisser S. Geerts N.C. Kyvsgaard D.P. McManus T.E. Nash Z.S. Pawlowski • Etiology • Taeniosis in humans • Cysticercosis in animals and humans • Biology and systematics • Epidemiology and geographical distribution • Diagnosis and treatment in humans • Detection in cattle and swine • Surveillance • Prevention • Control • Methods All OIE (World Organisation for Animal Health) publications are protected by international copyright law. Extracts may be copied, reproduced, translated, adapted or published in journals, documents, books, electronic media and any other medium destined for the public, for information, educational or commercial purposes, provided prior written permission has been granted by the OIE. The designations and denominations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the OIE concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers and boundaries. The views expressed in signed articles are solely the responsibility of the authors. The mention of specific companies or products of manufacturers, whether or not these have been patented, does not imply that these have been endorsed or recommended by the OIE in preference to others of a similar nature that are not mentioned. –––––––––– The designations employed and the presentation of material in this publication do not imply the expression of any opinion whatsoever on the part of the Food and Agriculture Organization of the United Nations, the World Health Organization or the World Organisation for Animal Health concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. -

New Aspects of Human Trichinellosis: the Impact of New Trichinella Species F Bruschi, K D Murrell
15 REVIEW Postgrad Med J: first published as 10.1136/pmj.78.915.15 on 1 January 2002. Downloaded from New aspects of human trichinellosis: the impact of new Trichinella species F Bruschi, K D Murrell ............................................................................................................................. Postgrad Med J 2002;78:15–22 Trichinellosis is a re-emerging zoonosis and more on anti-inflammatory drugs and antihelminthics clinical awareness is needed. In particular, the such as mebendazole and albendazole; the use of these drugs is now aided by greater clinical description of new Trichinella species such as T papuae experience with trichinellosis associated with the and T murrelli and the occurrence of human cases increased number of outbreaks. caused by T pseudospiralis, until very recently thought to The description of new Trichinella species, such as T murrelli and T papuae, as well as the occur only in animals, requires changes in our handling occurrence of outbreaks caused by species not of clinical trichinellosis, because existing knowledge is previously recognised as infective for humans, based mostly on cases due to classical T spiralis such as T pseudospiralis, now render the clinical picture of trichinellosis potentially more compli- infection. The aim of the present review is to integrate cated. Clinicians and particularly infectious dis- the experiences derived from different outbreaks around ease specialists should consider the issues dis- the world, caused by different Trichinella species, in cussed in this review when making a diagnosis and choosing treatment. order to provide a more comprehensive approach to diagnosis and treatment. SYSTEMATICS .......................................................................... Trichinellosis results from infection by a parasitic nematode belonging to the genus trichinella. -

Epidemiology of Angiostrongylus Cantonensis and Eosinophilic Meningitis
Epidemiology of Angiostrongylus cantonensis and eosinophilic meningitis in the People’s Republic of China INAUGURALDISSERTATION zur Erlangung der Würde eines Doktors der Philosophie vorgelegt der Philosophisch-Naturwissenschaftlichen Fakultät der Universität Basel von Shan Lv aus Xinyang, der Volksrepublik China Basel, 2011 Genehmigt von der Philosophisch-Naturwissenschaftlichen Fakult¨at auf Antrag von Prof. Dr. Jürg Utzinger, Prof. Dr. Peter Deplazes, Prof. Dr. Xiao-Nong Zhou, und Dr. Peter Steinmann Basel, den 21. Juni 2011 Prof. Dr. Martin Spiess Dekan der Philosophisch- Naturwissenschaftlichen Fakultät To my family Table of contents Table of contents Acknowledgements 1 Summary 5 Zusammenfassung 9 Figure index 13 Table index 15 1. Introduction 17 1.1. Life cycle of Angiostrongylus cantonensis 17 1.2. Angiostrongyliasis and eosinophilic meningitis 19 1.2.1. Clinical manifestation 19 1.2.2. Diagnosis 20 1.2.3. Treatment and clinical management 22 1.3. Global distribution and epidemiology 22 1.3.1. The origin 22 1.3.2. Global spread with emphasis on human activities 23 1.3.3. The epidemiology of angiostrongyliasis 26 1.4. Epidemiology of angiostrongyliasis in P.R. China 28 1.4.1. Emerging angiostrongyliasis with particular consideration to outbreaks and exotic snail species 28 1.4.2. Known endemic areas and host species 29 1.4.3. Risk factors associated with culture and socioeconomics 33 1.4.4. Research and control priorities 35 1.5. References 37 2. Goal and objectives 47 2.1. Goal 47 2.2. Objectives 47 I Table of contents 3. Human angiostrongyliasis outbreak in Dali, China 49 3.1. Abstract 50 3.2. -

Imaging Parasitic Diseases
Insights Imaging (2017) 8:101–125 DOI 10.1007/s13244-016-0525-2 REVIEW Unexpected hosts: imaging parasitic diseases Pablo Rodríguez Carnero1 & Paula Hernández Mateo2 & Susana Martín-Garre2 & Ángela García Pérez3 & Lourdes del Campo1 Received: 8 June 2016 /Revised: 8 September 2016 /Accepted: 28 September 2016 /Published online: 23 November 2016 # The Author(s) 2016. This article is published with open access at Springerlink.com Abstract Radiologists seldom encounter parasitic dis- • Some parasitic diseases are still endemic in certain regions eases in their daily practice in most of Europe, although in Europe. the incidence of these diseases is increasing due to mi- • Parasitic diseases can have complex life cycles often involv- gration and tourism from/to endemic areas. Moreover, ing different hosts. some parasitic diseases are still endemic in certain • Prompt diagnosis and treatment is essential for patient man- European regions, and immunocompromised individuals agement in parasitic diseases. also pose a higher risk of developing these conditions. • Radiologists should be able to recognise and suspect the This article reviews and summarises the imaging find- most relevant parasitic diseases. ings of some of the most important and frequent human parasitic diseases, including information about the para- Keywords Parasitic diseases . Radiology . Ultrasound . site’s life cycle, pathophysiology, clinical findings, diag- Multidetector computed tomography . Magnetic resonance nosis, and treatment. We include malaria, amoebiasis, imaging toxoplasmosis, trypanosomiasis, leishmaniasis, echino- coccosis, cysticercosis, clonorchiasis, schistosomiasis, fascioliasis, ascariasis, anisakiasis, dracunculiasis, and Introduction strongyloidiasis. The aim of this review is to help radi- ologists when dealing with these diseases or in cases Parasites are organisms that live in another organism at the where they are suspected. -

"Structure, Function and Evolution of the Nematode Genome"
Structure, Function and Advanced article Evolution of The Article Contents . Introduction Nematode Genome . Main Text Online posting date: 15th February 2013 Christian Ro¨delsperger, Max Planck Institute for Developmental Biology, Tuebingen, Germany Adrian Streit, Max Planck Institute for Developmental Biology, Tuebingen, Germany Ralf J Sommer, Max Planck Institute for Developmental Biology, Tuebingen, Germany In the past few years, an increasing number of draft gen- numerous variations. In some instances, multiple alter- ome sequences of multiple free-living and parasitic native forms for particular developmental stages exist, nematodes have been published. Although nematode most notably dauer juveniles, an alternative third juvenile genomes vary in size within an order of magnitude, com- stage capable of surviving long periods of starvation and other adverse conditions. Some or all stages can be para- pared with mammalian genomes, they are all very small. sitic (Anderson, 2000; Community; Eckert et al., 2005; Nevertheless, nematodes possess only marginally fewer Riddle et al., 1997). The minimal generation times and the genes than mammals do. Nematode genomes are very life expectancies vary greatly among nematodes and range compact and therefore form a highly attractive system for from a few days to several years. comparative studies of genome structure and evolution. Among the nematodes, numerous parasites of plants and Strikingly, approximately one-third of the genes in every animals, including man are of great medical and economic sequenced nematode genome has no recognisable importance (Lee, 2002). From phylogenetic analyses, it can homologues outside their genus. One observes high rates be concluded that parasitic life styles evolved at least seven of gene losses and gains, among them numerous examples times independently within the nematodes (four times with of gene acquisition by horizontal gene transfer. -

Report of the WHO Expert Consultation on Foodborne Trematode Infections and Taeniasis/Cysticercosis
Report of the WHO Expert Consultation on Foodborne Trematode Infections and Taeniasis/Cysticercosis Vientiane, Lao People's Democratic Republic 12-16 October 2009 © World Health Organization 2011 All rights reserved. Publications of the World Health Organization can be obtained from WHO Press, World Health Organization, 20 Avenue Appia, 1211 Geneva 27, Switzerland (tel.: +41 22 791 3264; fax: +41 22 791 4857; e-mail: [email protected]). Requests for permission to reproduce or translate WHO publications – whether for sale or for noncommercial distribution – should be addressed to WHO Press, at the above address (fax: +41 22 791 4806; e-mail: [email protected]). The designations employed and the presentation of the material in this publication do not imply the expression of any opinion whatsoever on the part of the World Health Organization concerning the legal status of any country, territory, city or area or of its authorities, or concerning the delimitation of its frontiers or boundaries. Dotted lines on maps represent approximate border lines for which there may not yet be full agreement. The mention of specific companies or of certain manufacturers’ products does not imply that they are endorsed or recommended by the World Health Organization in preference to others of a similar nature that are not mentioned. Errors and omissions excepted, the names of proprietary products are distinguished by initial capital letters. All reasonable precautions have been taken by the World Health Organization to verify the information contained in this publication. However, the published material is being distributed without warranty of any kind, either expressed or implied. The responsibility for the interpretation and use of the material lies with the reader. -

Trichinellosis Surveillance — United States, 2002–2007
Morbidity and Mortality Weekly Report www.cdc.gov/mmwr Surveillance Summaries December 4, 2009 / Vol. 58 / No. SS-9 Trichinellosis Surveillance — United States, 2002–2007 Department Of Health And Human Services Centers for Disease Control and Prevention MMWR CONTENTS The MMWR series of publications is published by Surveillance, Epidemiology, and Laboratory Services, Centers for Disease Control Introduction .............................................................................. 2 and Prevention (CDC), U.S. Department of Health and Human Methods ................................................................................... 2 Services, Atlanta, GA 30333. Results ...................................................................................... 2 Suggested Citation: Centers for Disease Control and Prevention. [Title]. Surveillance Summaries, [Date]. MMWR 2009;58(No. SS-#). Discussion................................................................................. 5 Centers for Disease Control and Prevention Conclusion ................................................................................ 7 Thomas R. Frieden, MD, MPH References ................................................................................ 7 Director Appendix ................................................................................. 8 Peter A. Briss, MD, MPH Acting Associate Director for Science James W. Stephens, PhD Office of the Associate Director for Science Stephen B. Thacker, MD, MSc Acting Deputy Director for Surveillance, Epidemiology, -

Taenia Solium Taeniosis/Cysticercosis and the Co-Distribution with Schistosomiasis in Africa Uffe Christian Braae1*, Christopher F
Braae et al. Parasites & Vectors (2015) 8:323 DOI 10.1186/s13071-015-0938-7 RESEARCH Open Access Taenia solium taeniosis/cysticercosis and the co-distribution with schistosomiasis in Africa Uffe Christian Braae1*, Christopher F. L. Saarnak1, Samson Mukaratirwa2, Brecht Devleesschauwer3,4, Pascal Magnussen1,5 and Maria Vang Johansen1 Abstract Background: This study aimed to map the distribution of Taenia solium taeniosis/cysticercosis and the co-distribution with schistosomiasis in Africa. These two major neglected tropical diseases are presumed to be widely distributed in Africa, but currently the level of co-distribution is unclear. Methods: A literature search on T. solium taeniosis/cysticercosis was performed to compile all known studies on the presence of T. solium and apparent prevalence of taeniosis and porcine cysticercosis in Africa. Studies were geo-referenced using an online gazetteer. A Bayesian framework was used to combine the epidemiological data on the apparent prevalence with external information on test characteristics to estimate informed district-level prevalence of taeniosis and porcine cysticercosis. Districts with T. solium taeniosis/cysticercosis presence were cross-referenced with the Global Neglected Tropical Diseases Database for schistosomiasis presence. Results: The search strategies identified 141 reports of T. solium in Africa from 1985 to 2014 from a total of 476 districts in 29 countries, 20 with porcine cysticercosis, 22 with human cysticercosis, and 16 with taeniosis, in addition to 2 countries identified from OIE reports. All 31 countries were considered, on national scale, to have co-distribution with schistosomiasis. Presence of both parasites was confirmed in 124 districts in 17 countries. The informed prevalence of taeniosis and porcine cysticercosis were estimated for 14 and 41 districts in 10 and 13 countries, respectively.