Role of Nattrassia Sp. in Fruit Orchard Decline And
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Muzaffargarh
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !! ! ! ! ! ! Overview - Muzaffargarh ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Bhattiwala Kherawala !Molewala Siwagwala ! Mari PuadhiMari Poadhi LelahLeiah ! ! Chanawala ! ! ! ! ! ! ! Ladhranwala Kherawala! ! ! ! Lerah Tindawala Ahmad Chirawala Bhukwala Jhang Tehsil ! ! ! ! ! ! ! Lalwala ! Pehar MorjhangiMarjhangi Anwarwal!a Khairewala ! ! ! ! ! ! ! ! ! Wali Dadwala MuhammadwalaJindawala Faqirewala ! ! ! ! ! ! ! ! ! MalkaniRetra !Shah Alamwala ! Bhindwalwala ! ! ! ! ! Patti Khar ! ! ! Dargaiwala Shah Alamwala ! ! ! ! ! ! Sultanwala ! ! Zubairwa(24e6)la Vasawa Khiarewala ! ! ! ! ! ! ! Jhok Bodo Mochiwala PakkaMochiwala KumharKumbar ! ! ! ! ! ! Qaziwala ! Haji MuhammadKhanwala Basti Dagi ! ! ! ! ! Lalwala Vasawa ! ! ! Mirani ! ! Munnawala! ! ! Mughlanwala ! Le! gend ! Sohnawala ! ! ! ! ! Pir Shahwala! ! ! Langanwala ! ! ! ! Chaubara ! Rajawala B!asti Saqi ! ! ! ! ! ! ! ! ! BuranawalaBuranawala !Gullanwala ! ! ! ! ! Jahaniawala ! ! ! ! ! Pathanwala Rajawala Maqaliwala Sanpalwala Massu Khanwala ! ! ! ! ! ! Bhandniwal!a Josawala ! ! Basti NasirBabhan Jaman Shah !Tarkhanwala ! !Mohanawala ! ! ! ! ! ! ! ! ! ! Basti Naseer Tarkhanwala Mohanawala !Citiy / Town ! Sohbawala ! Basti Bhedanwala ! ! ! ! ! ! Sohaganwala Bhurliwala ! ! ! ! Thattha BulaniBolani Ladhana Kunnal Thal Pharlawala ! ! ! ! ! ! ! ! ! ! ! Ganjiwala Pinglarwala Sanpal Siddiq Bajwa ! ! ! ! ! Anhiwala Balochanwala ! Pahrewali ! ! Ahmadwala ! ! ! -
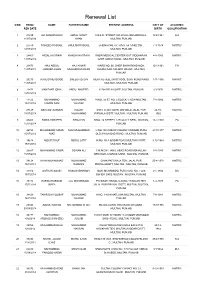
Renewal List
Renewal List S/NO REN# / NAME FATHER'S NAME PRESENT ADDRESS DATE OF ACADEMIC REN DATE BIRTH QUALIFICATION 1 21230 ALI AHMAD KHAN ABDUL MOHIT 122/8-M, STREET. NO.2CHAH BOHARWALA,, 14/4/1941 B.A 11/07/2014 KHAN MULTAN, PUNJAB 2 27570 NAHEED RASOOL GHULAM RASOOL GHUNGI NO. 07. H/NO. 64-A MULTAN , 7-9-1979 MATRIC 12/07/2014 MULTAN, PUNJAB 3 28469 AFZAL HUSSAIN KHADIM HUSSAIN SABIR MEDICAL CENTER OUT SIDEHARAM 4-8-1965 MATRIC 13/07/2014 GATE AKBAR ROAD , MULTAN, PUNJAB 4 21473 HAJI ABDUL HAJI KHAIR WARD NO. 06, SHER SHAH ROAD MOH, 10/1/1961 F.A 14/07/2014 HAMEED KHAN MUHAMMAD KHAN KHUDA DAD COLONY JOHAR , MULTAN, PUNJAB 5 26275 KHALID MEHBOOB SAEED-UD-DIN NEAR AL-JEELUN SCHOOL SURJ KUND RAOD 1-11-1966 MATRIC 14/07/2014 MULTAN , MULTAN, PUNJAB 6 21471 MANZHAR IQBAL ABDUL MAJEED H 764 DELHI GATE, MULTAN, PUNJAB 1/1/1970 MATRIC 14/07/2014 7 41123 MUHAMMAD MUHAMMAD H/NO. 32 ST, NO. 2 BLOCK Y NEW MULTAN, 7-8-1982 MATRIC 15/7/2014 USMAN SANI YOUSAF MULTAN, PUNJAB 8 27128 ASHFAQ HUSSAIN NAZAR MOH, CHAH DARKHAN WALA JALAL PUR 24-10- MATRIC 15/07/2014 MUHAMMAD PIRWALA DISTT, MULTAN , MULTAN, PUNJAB 1962 9 48463 RABIA PARVEEN SIRAJ DIN H/NO. 14 STREET, C BLOCK Y NEW , MULTAN, 16-2-1985 FA 15/07/2014 PUNJAB 10 46725 MUHAMMAD IMRAN NIAZ MUHAMMAD H NO 102 ZUBAIR COLONY FAROOQ PURA 21/7/1977 MATRIC 15/07/2014 NIAZ OLD SHUJA BAD ROAD , MULTAN, PUNJAB 11 39614 ABDUR RAUF ABDUL LATIF H/NO. -

Punjab Tourism for Economic Growth Final Report Consortium for Development Policy Research
Punjab Tourism for Economic Growth Final Report Consortium for Development Policy Research ABSTRACT This report documents the technical support provided by the Design Team, deployed by CDPR, and covers the recommendations for institutional and regulatory reforms as well as a proposed private sector participation framework for tourism sector in Punjab, in the context of religious tourism, to stimulate investment and economic growth. Pakistan: Cultural and Heritage Tourism Project ---------------------- (Back of the title page) ---------------------- This page is intentionally left blank. 2 Consortium for Development Policy Research Pakistan: Cultural and Heritage Tourism Project TABLE OF CONTENTS LIST OF ACRONYMS & ABBREVIATIONS 56 LIST OF FIGURES 78 LIST OF TABLES 89 LIST OF BOXES 910 ACKNOWLEDGMENTS 1011 EXECUTIVE SUMMARY 1112 1 BACKGROUND AND CONTEXT 1819 1.1 INTRODUCTION 1819 1.2 PAKISTAN’S TOURISM SECTOR 1819 1.3 TRAVEL AND TOURISM COMPETITIVENESS 2324 1.4 ECONOMIC POTENTIAL OF TOURISM SECTOR 2526 1.4.1 INTERNATIONAL TOURISM 2526 1.4.2 DOMESTIC TOURISM 2627 1.5 ECONOMIC POTENTIAL HERITAGE / RELIGIOUS TOURISM 2728 1.5.1 SIKH TOURISM - A CASE STUDY 2930 1.5.2 BUDDHIST TOURISM - A CASE STUDY 3536 1.6 DEVELOPING TOURISM - KEY ISSUES & CHALLENGES 3738 1.6.1 CHALLENGES FACED BY TOURISM SECTOR IN PUNJAB 3738 1.6.2 CHALLENGES SPECIFIC TO HERITAGE TOURISM 3940 2 EXISTING INSTITUTIONAL ARRANGEMENTS & REGULATORY FRAMEWORK FOR TOURISM SECTOR 4344 2.1 CURRENT INSTITUTIONAL ARRANGEMENTS 4344 2.1.1 YOUTH AFFAIRS, SPORTS, ARCHAEOLOGY AND TOURISM -

District SARGODHA Tehsil Group Batch No. Frist Name Last Name
District SARGODHA Tehsil Group Batch No. Frist Name Last Name Gender Teacher Type Cell No. Email EMIS Code School Name BHALWAL Arts Batch 1 Aamir Hayat Male Public 3016946997 [email protected] 38410151 GPS SULTAN PUR NOON BHALWAL Arts Batch 1 Aasia Sultan Female Public 3044644508 [email protected] 38410042 GGHSS LALLIANI BHALWAL Arts Batch 1 Aasma Javed Female Public 3069607018 [email protected] 38410503 GGES CHAK NO.16 NB BHALWAL Arts Batch 1 Abdur Rehman Male Public 3075332038 [email protected] 38410013 GHS CHAK NO.22 NB BHALWAL Arts Batch 1 Abida Mumtaz Female Public 3474800449 [email protected] 38410040 GGHSS CHAK NO.10 ML BHALWAL Arts Batch 1 Abida Naseem Female Private 0303-4247331 [email protected] 384107406 GHAZALI MODEL HIGH SCHOOL BOYS PHULARWAN BHALWAL Arts Batch 1 Abida Rani Female Public 3447475290 [email protected] 38410068 GES PHULARWAN BHALWAL Arts Batch 1 Abida Tasneem Female Public 3434346283 [email protected] 38410187 GMPS CHAK NO.14 SB PATHAN WALA BHALWAL Arts Batch 1 Adeeba Javaid Female Public 3414300718 [email protected] 38410331 GMPS PHULARWAN KOHNA BHALWAL Arts Batch 1 Adeela Nawaz Female Private 0301-6792573 [email protected] other other BHALWAL Arts Batch 1 Afsana Kanwal Female Public 3327546064 [email protected] 38410681 GGPS AHLI DAKHLI 4 NB BHALWAL Arts Batch 1 Afshan Batool Female Public 3417977817 [email protected] 38410505 GGPS DERA BACHIAN WALA BHALWAL Arts Batch 1 Afshan Noreen Female Private 0306-8604984 [email protected] -

33422717.Pdf
1 Contents 1. PREFACE ........................................................................................................................................... 4 2. OVERVIEW OF THE CULTURAL ASSETS OF THE COMMUNITIES OF DISTRICTS MULTAN AND BAHAWALPUR ................................................................... 9 3. THE CAPITAL CITY OF BAHAWALPUR AND ITS ARCHITECTURE ............................ 45 4. THE DECORATIVE BUILDING ARTS ....................................................................................... 95 5. THE ODES OF CHOLISTAN DESERT ....................................................................................... 145 6. THE VIBRANT HERITAGE OF THE TRADITIONAL TEXTILE CRAFTS ..................... 165 7. NARRATIVES ................................................................................................................................... 193 8. AnnEX .............................................................................................................................................. 206 9. GlossARY OF TERMS ................................................................................................................ 226 10. BIBLIOGRAPHY ............................................................................................................................. 234 11. REPORTS .......................................................................................................................................... 237 12 CONTRibutoRS ............................................................................................................................ -

Chief Minister Self Employment Scheme for Unemployed Educated Youth
Winner List Chief Minister Self Employment Scheme for Unemployed Educated Youth Multan Division NIC ApplicantName GuardianName Address WinOrder Distt. Khanewal Jahanian (Bolan) Key Used: aghakhurm 3610102439763 ABDUL HAFEEZ M. HANIF 105/10R JAHANIAN DIST. 1 3610102595791 LIAQAT HUSSAIN ABDUL HAMEED THATA SADIQABAD, TEH JAHANIA, 2 DISTT. KHANWALA 3610102707797 GHAZANFAR HASSAN MOHAMMAD SHAKAR H NO. 225, BLOCK NO. 5, JAHANIAN 3 DISTT. KHANEWAL. 3610180513779 WASEEM ALI M. SARWAR CHAK NO. 121/10-R TEH. JAHANIA 4 DISTT. KHANEWAL 3520227188361 MOHAMMAD JAVED MOHAMMAD SHAFI FLAT NO 752C BLOCK Q MODEL TOWN 5 LAHORE 3610141795247 Muhammad Shahid Shahzada Zahid Raza Rahim Shah Road H/NO.164/A Jinnah 6 Abdil Jahanian D 3610132117859 MUHAMMAD DILSHAD ALI JAMSHAD ALI CHAK NO. 135/10R, TEHSEEL 7 JAHANIAN DISTT. KHANEWAL 3610102391383 TAHIR ABBAS AZIZ AHMED CHAK KHRIA POST 99-10R RAHEEM 8 SHAH 3610180588801 SOHAIL IJAZ IAJZ AHMED CHAK NO 116/10-R NEW TEH 9 JAHANIAN KHANEWAL 3610112010659 MOHAMMAD RIAZ FALIK SHER CHAK NO 157/10-R P/O JUNGLE 10 MARYALA JHASIL JAHANIA 3610166557189 MUHAMMAD THIR MUHAMMAD SLEEM CAHK NO 135/10R 11 3610171420543 TOQEER HUSSAIN SAEED SAEED AKHTAR CHAK NO. 132/10R, P.O. THATHA 12 SADIQ ABAD TEH JHANI 3610102461071 MUHAMMAD NASRULLAH KHUSHI MUHAMMAD BLOCK # 4 JAHANIAN KWL 13 3610181422213 HAFIZ ASIF JAVED ABDUL GHAFAR CHAK NO. 107/10-R TEH. JAHANIAN 14 DISTT. KHANEWAL 3610115748627 MUHAMMAD ASIF MUHAMMAD RAMZAN CHAK NO 114-10R JAHIANIAN DIST 15 KHANEWAL 3610154828857 ASIF BASHIR BASHIR AHMAD MAMTAZ LAKR MANDI TEH. JAHANIAN DISTT. 16 KHANEWAL 3610102391373 MUHAMMAD SARWAR MUHAMMAD NAZIR AHMED OPP BHATTI SERVICE STATION SIAL 17 TOWN JHN TEH JHN D 3610102750511 ASIF ISMAIL LIAQAT ALI CH. -

Persecution of Ahmadis in Pakista During the Year 2000
Persecution of Ahmadis in Pakistan during the Year 2000 A Summary CONTENTS Ø Foreword Ø The Slaughter at Ghatialian Ø The Gory Riot of Takht Hazara Ø Other Murders Ø Physical Assaults Ø Mosques Targeted Ø The Evil of the Anti-terrorism Act Ø Endless Prosecution on Religious Basis Ø Prisoners of Conscience Ø The Official Position Ø The Role of Judiciary Ø Tension and Insecurity all over Ø A Fuller Report from Azad Kashmir Ø Rabwah remains a Prime Target Ø Miscellaneous Ø The Year of Human Rights and Dignity! Ø Conclusion Annexes 1. Details of Cases Registered against Ahmadis during the year 2000 2. Five Important Cases - Special Mention 3. An Outrageous Handout 4. An Outline of Persecution of Ahmadis in Pakistan Some Statistics and Information – 2000 Page 1 Newsreport - Year 2000 - Summary http://www.ThePersecution.org/ Foreword Only 11 weeks prior to ringing in the New Year 2000 the military regime took over in Islamabad, and General Musharraf spoke the brave words on 17 October 1999 that all citizens were equal in Pakistan. This was a whiff of fresh air after a long time, and Ahmadis hoped that it would change into a breeze of freedom and human rights not only for them but for other marginalized sections of the society. Alas, that was not to be. The military regime soon succumbed to the pressure tactics of clerics. Before the New Year arrived, the regime opted on the National Security Council, Mr. Mahmood Ghazi, an anti-Ahmadi cleric of long standing. A mulla-led mob attacked the under-construction house at Okara of the District President of the Ahmadiyya Community, demolished the new-construction, looted the old house and set it on fire in the presence of authorities who later shamelessly arrested the victim and his two sons and registered a case against them under the anti-Ahmadiyya law, while none of the rioters or their leaders was taken to task. -

Mali Sargodha District.Pdf
1 LIST OF CANDIDATES FOR THE POST OF MALI DISTRICT SARGODHA APPLICATI AGE AS ON Mark Mark Total NEW SR. NO. FORM NO. NAME FATHER NAME GENDER RELEG: CNIC NO. DOB 7 Apr 2021 ADDRESS DOMICILE DISTRICT EDU: MOB NO QUOTA EXPERIENCE DOCUMENT STATUS REMARKS Merit ON ID NO. Y M D Obtain Obtain Marks 1 1 98 M. Riaz Male Islam 38403-8185100-5 9-Apr-01 19 11 28 Faisal Town, Streeet No. 4 Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not ed in ed in M. Zeeshan 0301-1478924 Attached Test Intervi 2 2 49 M. Riaz Male Islam 38403-9309144-3 26-Sep-98 22 6 11 Chak No. 53, Janoobi Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not Mohsin Riaz ew 0343-8012791 Attached 3 3 50 M. Ramzan Male Islam 38402-0120365-1 28-Feb-93 28 1 9 Asar Sarkharo, P.O Dharwal, Sargodha Sargodha Matric Open Nil REJECTED Affidavit & Experience Not M. Shakeel 0304-9120648 Attached 4 4 61 Akram Masih Male Christian 38403-5606473-5 10-Jan-00 21 2 27 Chak No. 43, Giyanwala P.O Sargodha Sargodha Middle Open Nil REJECTED Affidavit & Experience Not Shehroon Masih Nil Attached 5 5 80 Nasir Hameed Male Islam 38403-7925256-1 25-Aug-98 22 7 12 House No. 9, P.T.S Sargodha Sargodha Middle Open Nil REJECTED Affidavit & Experience Not Sheraz Baloch 0308-1209491 Attached 6 6 104 M.aslam Male Islam 38403-7222978-5 22-Oct-00 20 5 15 Johar Colony House no. 2 Mohallah Sargodha Matric Open Nil REJECTED Affidavit & Experience Not M. -

Abstract 1924, but the Sargodha District Khilafat Committee Continued Its Functioning
URL: http://dx.doi.org/10.31703/gpr.2020(V-IV).12 DOI: 10.31703/gpr.2020(V-IV).12 Citation: Pervez, M., Dasti, H. A., & Khan, A. R. (2021). The Emergence of the Khilafat Movement in Sargodha: Beginning of Agitational Politics and Impacts on the Freedom Movement. Global Political Review, V(IV), 105-112. https://doi.org/10.31703/gpr.2020(V-IV).12 Vol. V, No. IV (Fall 2020) Pages: 105 – 112 The Emergence of the Khilafat Movement in Sargodha: Beginning of Agitational Politics and Impacts on the Freedom Movement Muhammad Pervez* Humaira Arif Dasti† Abdul Rasheed Khan‡ p- ISSN: 2521-2982 e- ISSN: 2707-4587 Mustafa Kamal abolished the institution of Khilafat in p- ISSN: 2521-2982 Abstract 1924, but the Sargodha district Khilafat Committee continued its functioning. Peers of Sial Sharif and Bugvis of Bhera played the leading role, while pro-British feudal lords supported the government. Headings Khilafat conferences were held in which high-level Khilafat leaders participated and addressed. Samarna-Fund was collected, and a sum • Introduction of Rs 9600 was submitted to the Punjab Khilafat Committee. Sialvi and • Method and, Material Bugvi visited different towns and villages and conveyed the Khilafat • Backwardness of Sargodha message in simple words. Bugvi was arrested and sent to prison for one • Disunity and Weakness and a half year. While on the arrest of Peer of Sial-Sharif, the Muslims • Participation of Muslims of Sargodha of Soon Valley began to offer arrest every day. From the Khilafat in the Khilafat Movement movement, Muslims of Sargodha enhanced their awareness and gained • Sargodha under British Rule experience. -

Multan District 429,186 285,623 72,505 71,058 398,178 405,853
TABLE -24 SELECTED HOUSING CHARACTERISTICS OF RURAL LOCALITIES HOUSING CHARACTERISTICS HADBAST HOUSING FACILITIES AVERAGE NAME OF MAUZA / DEH NUMBER / TYPE OF HOUSING UNIT HOUSEHOL / VILLAGE / SETTLMENT DEH POTABLE D SIZE NUMBER ELECTRICITY GAS KITCHEN BATH ROOM LATRINE TOTAL PACCA SEMI PACCA KACHA WATER 1 2 3 4 5 6 7 8 9 10 11 12 13 MULTAN DISTRICT 429,186 285,623 72,505 71,058 398,178 405,853 76,353 194,718 298,791 324,999 6.20 JALALPUR PIRWALA TEHSIL 78,875 46,262 19,157 13,456 69,060 70,792 2,113 29,612 41,099 48,223 6.14 ABADI JALALPUR PIRWALA QH 14,525 8,331 3,679 2,515 8,522 12,263 116 6,089 7,363 8,015 5.84 060/M PC 467 249 98 120 183 419 - 51 146 148 5.93 CHAK NO 060/M 0000074 189 33 91 65 175 186 - 36 41 41 5.93 CHAK NO 061/M 0000072 67 53 5 9 8 48 - 5 30 31 6.03 CHAK NO 062/M 0000073 211 163 2 46 - 185 - 10 75 76 5.91 063/M PC 451 241 162 48 355 403 2 133 124 140 5.67 CHAK NO 063/M 0000071 213 136 61 16 199 177 1 68 61 76 5.50 CHAK NO 064/M 0000100 138 64 62 12 135 133 1 9 8 7 5.54 CHAK NO 066/M 0000099 100 41 39 20 21 93 - 56 55 57 6.21 067/M PC 1,357 599 485 273 405 1,113 - 181 346 543 5.80 CHAK NO 067/M 0000097 371 236 71 64 128 288 - 53 145 162 6.50 CHAK NO 068/M 0000096 364 103 183 78 56 292 - 10 44 75 4.86 CHAK NO 069/M 0000098 326 70 180 76 3 255 - 47 69 170 5.15 CHAK NO 070/M 0000104 180 98 35 47 128 168 - 34 38 76 6.25 CHAK NO 071/M 0000103 116 92 16 8 90 110 - 37 50 60 7.63 072/M PC 418 294 82 42 179 259 42 145 158 135 5.67 CHAK NO 065/M 0000101 150 126 9 15 77 84 - 19 26 27 5.72 CHAK NO 072/M 0000102 167 101 57 9 -

Audit Report on the Accounts of Town Municipaladministrations City District Multan
AUDIT REPORT ON THE ACCOUNTS OF TOWN MUNICIPALADMINISTRATIONS CITY DISTRICT MULTAN AUDITYEAR 2013-2014 AUDITOR GENERAL OF PAKISTAN TABLE OF CONTENTS ABBREVIATIONS AND ACRONYMS ....................................................................... i PREFACE ....................................................................................................................... ii EXECUTIVE SUMMARY ...........................................................................................iii SUMMARY TABLES AND CHARTS .....................................................................viii Table 1: Audit Work Statistics ..................................................................................................... viii Table 2: Audit Observations regarding Financial Management ............................................... viii Table 3: Outcome Statistics ............................................................................................................ ix Table 4: Irregularities Pointed Out .................................................................................................. x Table 5: Cost -Benefit ...................................................................................................................... x CHAPTER-1 ................................................................................................................... 1 1. TOWN MUNICIPAL ADMINISTRATIONS, CITY DISTRICT MULTAN ............ 1 1.1 INTRODUCTION .............................................................................................................. -

Punjab ! ! Overview ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! !
! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! - PUNJAB ! ! OVERVIEW ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! KHYBER ! ! ! ! ! PAKHTUNKHWA ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! ! Chamba Pind ! ! ! Attock ! ! ! Hazro ! ! ! Murree ! ! ! ! Bhabra PAK ! ! ! ! ! AttockBura Hassan Abdal ! ! Wah ! Amanpura ! ! ! ! !!! ! Kotli Sattian ! A!ttock ! Bhangal ! Taxila ! ! FATA Akhori Bahlol ! ! ! ! Autrinna Mariala Bhatiot Badhana Kalan ! ! ! ! ! Rawalpindi ! ! ! ! ! !! Rawalpindi Kahuta ! Fatehjang ! ! ! Basal ! ! Morgah JAMMU & KASHMIR ! Jalwal Bango ! ! Achhral ! Band ! ! Murat ! ! Rangli Fateh Jang ! ! ATTOCK ! Adiala Gali Jagir ! Jand Abawal Bagh ! ! Bhunan Wali ! Dulehal ! ! Kallar Sayedan ! ! ! ! Bagra Arazi Chhur Mall Choha Khalsa ! ! Rawalpindi ! Jand Mandra ! !Ghalwal ! Dhok Ganganwali ! ! Malikpur ! Pari Kali ! Jhamat Dabhula ! ! Malangi ! ! Ahmadal Balawal ! ! ! ! ! ! RAWALPINDI Gujar Khan ! Saura ! Ratala ! ! Pindi Gheb ! ! Chak Beli ! !Pindi Gheb !Maghian ! Gujar Khan ! ! Malal ! ! Maira Behkhari ! ! Neela ! ! Hadawali ! ! ! Bahwaley Kallan Dora Badhal ! Dhok Afghan ! Adhi ! Makhad ! Visor ! ! ! Banth Pandori ! ! ! Dhok Abakki ! ! ! ! Shah Muhammad Wali Dewal Jamalwal Dhudial Arazi Hamid ! Bor Khui ! ! ! ! Hasola ! ! ! Multan ! !