Neurosteroids As Novel Antidepressants and Anxiolytics: GABA-A Receptors and Beyond
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Zuranolone | Medchemexpress
Inhibitors Product Data Sheet Zuranolone • Agonists Cat. No.: HY-103040 CAS No.: 1632051-40-1 Molecular Formula: C₂₅H₃₅N₃O₂ • Molecular Weight: 409.56 Screening Libraries Target: GABA Receptor Pathway: Membrane Transporter/Ion Channel; Neuronal Signaling Storage: Powder -20°C 3 years 4°C 2 years In solvent -80°C 6 months -20°C 1 month SOLVENT & SOLUBILITY In Vitro DMSO : 100 mg/mL (244.16 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) Mass Solvent 1 mg 5 mg 10 mg Concentration Preparing 1 mM 2.4416 mL 12.2082 mL 24.4164 mL Stock Solutions 5 mM 0.4883 mL 2.4416 mL 4.8833 mL 10 mM 0.2442 mL 1.2208 mL 2.4416 mL Please refer to the solubility information to select the appropriate solvent. In Vivo 1. Add each solvent one by one: 10% DMSO >> 40% PEG300 >> 5% Tween-80 >> 45% saline Solubility: 2.5 mg/mL (6.10 mM); Suspended solution; Need ultrasonic 2. Add each solvent one by one: 10% DMSO >> 90% (20% SBE-β-CD in saline) Solubility: ≥ 2.5 mg/mL (6.10 mM); Clear solution 3. Add each solvent one by one: 10% DMSO >> 90% corn oil Solubility: ≥ 2.5 mg/mL (6.10 mM); Clear solution 4. Add each solvent one by one: 5% DMSO >> 40% PEG300 >> 5% Tween-80 >> 50% saline Solubility: 2.5 mg/mL (6.10 mM); Suspended solution; Need ultrasonic 5. Add each solvent one by one: 5% DMSO >> 95% (20% SBE-β-CD in saline) Solubility: ≥ 2.5 mg/mL (6.10 mM); Clear solution BIOLOGICAL ACTIVITY Description Zuranolone is an orally active and potent neuroactive steroid positive allosteric modulator of GABAA receptor, with EC50s of [1] 296 and 163 nM for α1β2γ2 and α4β3δ GABAA receptors, respectively . -
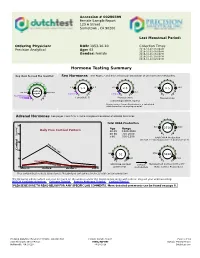
Hormone Testing Summary
Accession # 00280399 Female Sample Report 123 A Street Sometown , CA 90266 Last Menstrual Period: Ordering Physician: DOB: 1953-10-10 Collection Times: Precision Analytical Age: 63 2016-10-02 06:00AM 2016-10-02 08:00AM Gender: Female 2016-10-01 06:00PM 2016-10-01 10:00PM 2016-10-02 02:00AM Hormone Testing Summary Key (how to read the results): Sex Hormones See Pages 2 and 3 for a thorough breakdown of sex hormone metabolites opausa n l R e a m n e g 1.8 4.5 6.0 2.3 14.0 r 5.1 2.8 1.5 P e patient low limit high limit 20.0 result 0.2-0.7 0.3-2.0 Postmenopausal range Estradiol(E2) Progesterone Testosterone (Serum Equivalent, ng/mL) Progesterone Serum Equivalent is a calculated value based on urine pregnanediol. Adrenal Hormones See pages 4 and 5 for a more complete breakdown of adrenal hormones Total DHEA Production 300 500 3000 Age Range 2516 Daily Free Cortisol Pattern 20-39 1300-3000 240 40-60 750-2000 (ng/mg) >60 500-1200 Total DHEA Production (DHEAS + Etiocholanolone + Androsterone) 180 High Range Limit 120Cortisol 80 52 2750 5930 60 230 6500 Patient Values Low Range Limit 24hr Free Cortisol cortisol Metabolized Cortisol (THF+THE) 0 (A+B+C+D) metabolism (Total Cortisol Production) Waking (A) Morning (B) Afternoon (C) Night (D) Free cortisol best reflects tissue levels. Metabolized cortisol best reflects total cortisol production. The following videos (which can also be found on the website under the listed names along with others) may aid your understanding: DUTCH Complete Overview Estrogen Tutorial Female Androgen Tutorial Cortisol Tutorial PLEASE BE SURE TO READ BELOW FOR A NY SPECIFIC LA B COMMENTS. -

Seco-Steroids C07C)
CPC - C07J - 2021.08 C07J STEROIDS (seco-steroids C07C) Definition statement This place covers: Compounds containing a cyclopenta[a]hydrophenanthrene skeleton (see below) or a ring structure derived therefrom: • by contraction or expansion of one ring by one or two atoms; • by contraction or expansion of two rings each by one atom; • by contraction of one ring by one atom and expansion of one ring by one atom; • by substitution of one or two carbon atoms of the cyclopenta[a]hydrophenanthrene skeleton, which are not shared by rings, by hetero atoms, in combination with the above defined contraction or expansion or not, or; • by condensation with carbocyclic or heterocyclic rings in combination with one or more of the foregoing alterations or not. Preparation of steroids including purification, separation, stabilisation or use of additives unless provided for elsewhere, as specified below. Treatment and modification of steroids provided that • the treatment is not provided for elsewhere and • the resultant product is a compound under the subclass definition. Relationships with other classification places In class C07, in the absence of an indication to the contrary, a compound is classified in the last appropriate place, i.e. in the last appropriate subclass. For example cyclopenta [a] hydrophenantrenes are classified in subclass C07J as steroids and not in subclasses C07C or C07D as carbocyclic or heterocyclic compounds. Subclass C07J is a function-oriented entry for the compounds themselves and does not cover the application or use of the compounds under the subclass definition. For classifying such information other entries in the IPC exist, for example: Subclass A01N: Preservation of bodies of humans or animals or plants or parts thereof; biocides, e.g. -

Three-Dimensional Structure of Holo 3A,20J3-Hydroxysteroid
Proc. Nati. Acad. Sci. USA Vol. 88, pp. 10064-10068, November 1991 Biochemistry Three-dimensional structure of holo 3a,20j3-hydroxysteroid dehydrogenase: A member of a short-chain dehydrogenase family (x-ray crystaflography/steroid-metabolizing enzyme/dinucleotide-linked oxldoreductase/sterold-protein interaction/sequence and folding homologies) DEBASHIS GHOSH*t, CHARLES M. WEEKS*, PAWEL GROCHULSKI*t, WILLIAM L. DUAX*, MARY ERMAN*, ROBERT L. RIMSAY§, AND J. C. ORR§ *Medical Foundation of Buffalo, 73 High Street, Buffalo, NY 14203; and Memorial University of Newfoundland, St. John's, Newfoundland, Canada AlB 3V6 Communicated by Herbert A. Hauptman, July 18, 1991 (receivedfor review May 14, 1991) ABSTRACT The x-ray structure of a short-chain dehy- the substrate binding regions, offers further insight concern- drogenase, the bacterial holo 3a,20/3-hydroxysteroid dehydro- ing the significance of conserved residues and their possible genase (EC 1.1.1.53), is described at 2.6 A resolution. This roles in substrate specificity and overall enzyme function. enzyme is active as a tetramer and crystallizes with four identical subunits in the asymmetric unit. It has the a/( fold characteristic ofthe dinucleotide binding region. The fold ofthe MATERIALS AND METHODS rest of the subunit, the quarternary structure, and the nature The crystals, grown in the presence of 4 mM NADH, belong ofthe cofactor-enzyme interactions are, however, significantly to the space group P43212 having unit cell dimensions a = different from those observed in the long-chain dehydrogena- 106.2 A and c = 203.8 A and contain one full tetramer (106 ses. The architecture of the postulated active site is consistent kDa) in the asymmetric unit (13). -

Proceedings of the Thirtieth Annual Meeting of the American Society for Clinical Investigation Held in Atlantic City, N
PROCEEDINGS OF THE THIRTIETH ANNUAL MEETING OF THE AMERICAN SOCIETY FOR CLINICAL INVESTIGATION HELD IN ATLANTIC CITY, N. J., MAY 2, 1938 J Clin Invest. 1938;17(4):501-537. https://doi.org/10.1172/JCI100977. Research Article Find the latest version: https://jci.me/100977/pdf PROCEEDINGS OF THE THIRTIETH ANNUAL MEETING OF THE AMERICAN SOCIETY FOR CLINICAL INVESTIGATION HELD IN ATLANTIC CITY, N. J., MAY 2, 1938 READ BEFORE THE SCIENTIFIC SESSION The Successful Treatment of Pernicious Anemia by in powdered form hemostasis was readily obtained in Means of Non-Autolyzed Yeast. By MAXWELL M. hemorrhages following nine dental extractions and three WINTROBE, Baltimore, Md. external wounds in five hemophilic subjects. When ap- It has been the general opinion that yeast, if it pos- plied in liquid form as other hemostatics are usually em- sesses any antianemic potency whatever, is effective only 1 loyed the results were unsatisfactory. Since the co- after autolysis and then only by virtue of its content of agulation time of the circulating blood was unchanged the "extrinsic factor." The observations reported contradict effectiveness of powdered beef globulin substance when this view and indicate that dehydrated yeast which has locally applied to a bleeding wound in hemophilia is not been subjected to autolysis, contains an antiper- attributed to the rapid formation of a firm fibrin clot. nicious anemia substance. Yeast obtained from two dif- The failure of liquid preparations may be due to the ferent sources was effective in the treatment of classical inability to maintain a sufficient concentration of the cases of pernicious anemia. -

The Steroid Metabolome in Men with Mood and Anxiety Disorders
Physiol. Res. 64 (Suppl. 2): S275-S282, 2015 https://doi.org/10.33549/physiolres.933067 The Steroid Metabolome in Men With Mood and Anxiety Disorders M. DUŠKOVÁ1, M. HILL1, M. BIČÍKOVÁ1, M. ŠRÁMKOVÁ1, D. ŘÍPOVÁ2, P. MOHR2, L. STÁRKA1 1Institute of Endocrinology, Prague, Czech Republic, 2National Institute of Mental Health, Klecany, Czech Republic Received May 5, 2015 Accepted May 20, 2015 Summary many other hormones and various factors have been The mood and behavior of individuals result from an orchestra of identified as modulators of mood and behavior in such many factors. Among them steroids play an important role; disorders. Recent studies have described the role of brain- however, only several common hormones have been investigated derived neurotrophic factor (BDNF) (Pluchino et al. in this respect. It has been demonstrated that some steroid 2013, Numakawa et al. 2014), thyroid hormones (Duntas metabolites long considered merely the products of steroid and Mails 2013), inflammation (Halaris 2013), immunity hormone metabolism in fact possess considerable activity in the (Pitychoutis and Papadopoulou-Daifoti 2010), melatonin CNS. For this reason we studied the steroid metabolome (Boyce and Hopwood 2013), oxytocin and vasopressin including 50 analytes in 20 men with depression, 20 men with (Scantamburlo et al. 2009, Matsuzaki et al. 2012), the anxiety and 30 healthy controls. Significant differences were renin-angiotensin-aldosterone system (Franklin et al. found not only between controls and men with either depression 2012, Murck et al. 2012), the cannabinoid system or anxiety, but also between men with depression and anxiety. (Martykánová 2010, Gorzalka and Hill 2011, Smaga et Particularly striking were those steroids until now not generally al. -

GABA Receptors
D Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews • BIOTREND Reviews Review No.7 / 1-2011 GABA receptors Wolfgang Froestl , CNS & Chemistry Expert, AC Immune SA, PSE Building B - EPFL, CH-1015 Lausanne, Phone: +41 21 693 91 43, FAX: +41 21 693 91 20, E-mail: [email protected] GABA Activation of the GABA A receptor leads to an influx of chloride GABA ( -aminobutyric acid; Figure 1) is the most important and ions and to a hyperpolarization of the membrane. 16 subunits with γ most abundant inhibitory neurotransmitter in the mammalian molecular weights between 50 and 65 kD have been identified brain 1,2 , where it was first discovered in 1950 3-5 . It is a small achiral so far, 6 subunits, 3 subunits, 3 subunits, and the , , α β γ δ ε θ molecule with molecular weight of 103 g/mol and high water solu - and subunits 8,9 . π bility. At 25°C one gram of water can dissolve 1.3 grams of GABA. 2 Such a hydrophilic molecule (log P = -2.13, PSA = 63.3 Å ) cannot In the meantime all GABA A receptor binding sites have been eluci - cross the blood brain barrier. It is produced in the brain by decarb- dated in great detail. The GABA site is located at the interface oxylation of L-glutamic acid by the enzyme glutamic acid decarb- between and subunits. Benzodiazepines interact with subunit α β oxylase (GAD, EC 4.1.1.15). It is a neutral amino acid with pK = combinations ( ) ( ) , which is the most abundant combi - 1 α1 2 β2 2 γ2 4.23 and pK = 10.43. -

Dynamic Regulation of the GABAA Receptor Function by Redox Mechanisms S
Supplemental material to this article can be found at: http://molpharm.aspetjournals.org/content/suppl/2016/07/20/mol.116.105205.DC1 1521-0111/90/3/326–333$25.00 http://dx.doi.org/10.1124/mol.116.105205 MOLECULAR PHARMACOLOGY Mol Pharmacol 90:326–333, September 2016 Copyright ª 2016 by The American Society for Pharmacology and Experimental Therapeutics MINIREVIEW—A LATIN AMERICAN PERSPECTIVE ON ION CHANNELS Dynamic Regulation of the GABAA Receptor Function by Redox Mechanisms s Daniel J. Calvo and Andrea N. Beltrán González Laboratorio de Neurobiología Celular y Molecular, Instituto de Investigaciones en Ingeniería Genética y Biología Molecular Downloaded from ¨Dr. Héctor N. Torres¨ (INGEBI), Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET), Ciudad Autónoma de Buenos Aires, Argentina (D.J.C., A.N.B.G.) Received May 15, 2016; accepted July 14, 2016 ABSTRACT molpharm.aspetjournals.org Oxidizing and reducing agents, which are currently involved normally present in neurons and glia or are endogenously in cell metabolism and signaling pathways, can regulate fast generated in these cells under physiologic states or during inhibitory neurotransmission mediated by GABA receptors in the oxidative stress (e.g., hydrogen peroxide, superoxide and hy- nervous system. A number of in vitro studies have shown that droxyl radicals, nitric oxide, ascorbic acid, and glutathione), diverse redox compounds, including redox metabolites and induce potentiating or inhibiting actions on different native and reactive oxygen and nitrogen species, modulate phasic and recombinant GABAA receptor subtypes. Based on these results, it tonic responses mediated by neuronal GABAA receptors through is thought that redox signaling might represent a homeostatic both presynaptic and postsynaptic mechanisms. -

Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors
International Journal of Molecular Sciences Article Neonatal Clonazepam Administration Induced Long-Lasting Changes in GABAA and GABAB Receptors Hana Kubová 1,* , Zde ˇnkaBendová 2,3 , Simona Moravcová 2,3 , Dominika Paˇcesová 2,3, Luisa Rocha 4 and Pavel Mareš 1 1 Institute of Physiology, Academy of Sciences of the Czech Republic, 14220 Prague, Czech Republic; [email protected] 2 Faculty of Science, Charles University, 12800 Prague, Czech Republic; [email protected] (Z.B.); [email protected] (S.M.); [email protected] (D.P.) 3 National Institute of Mental Health, 25067 Klecany, Czech Republic 4 Pharmacobiology Department, Center of Research and Advanced Studies, Mexico City 14330, Mexico; [email protected] * Correspondence: [email protected]; Tel.: +420-2-4106-2565 Received: 31 March 2020; Accepted: 28 April 2020; Published: 30 April 2020 Abstract: Benzodiazepines (BZDs) are widely used in patients of all ages. Unlike adults, neonatal animals treated with BZDs exhibit a variety of behavioral deficits later in life; however, the mechanisms underlying these deficits are poorly understood. This study aims to examine whether administration of clonazepam (CZP; 1 mg/kg/day) in 7–11-day-old rats affects Gama aminobutyric acid (GABA)ergic receptors in both the short and long terms. Using RT-PCR and quantitative autoradiography, we examined the expression of the selected GABAA receptor subunits (α1, α2, α4, γ2, and δ) and the GABAB B2 subunit, and GABAA, benzodiazepine, and GABAB receptor binding 48 h, 1 week, and 2 months after treatment discontinuation. Within one week after CZP cessation, the expression of the α2 subunit was upregulated, whereas that of the δ subunit was downregulated in both the hippocampus and cortex. -

Materializing Estrogen and Regulation Under Canada's Food and Drugs Act, 1939-1953 Lara Jessie Tessaro
Osgoode Hall Law School of York University Osgoode Digital Commons LLM Theses Theses and Dissertations 8-27-2018 Toxic Enactments: Materializing Estrogen and Regulation Under Canada's Food and Drugs Act, 1939-1953 Lara Jessie Tessaro Follow this and additional works at: https://digitalcommons.osgoode.yorku.ca/llm Part of the Legal History Commons TOXIC ENACTMENTS: MATERIALIZING ESTROGEN AND REGULATION UNDER CANADA’S FOOD AND DRUGS ACT, 1939-1953 LARA TESSARO A THESIS SUBMITTED TO THE FACULTY OF GRADUATE STUDIES IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF LAWS GRADUATE PROGRAM IN LAW OSGOODE HALL LAW SCHOOL, YORK UNIVERSITY TORONTO, ONTARIO August 2018 © Lara Tessaro, 2018 ABSTRACT The study describes how estrogen was standardized in Canada, in the 1940s and early 1950s, under the Food and Drugs Act. Contributing to interdisciplinary conversations, it provides an empirical case of how regulatory practices enact material realities. Using archival material, the study describes how estrogen was achieved, in part, through heterogeneous practices of the Canadian Committee on Pharmacopoeial Standards, National Health, and government solicitors. These regulators disagreed on whether, how, and by whom estrogens should be standardized. Rather than resolve these disagreements, Canada enacted multiple regulations purporting to standardize estrogen, and government solicitors practiced “techniques of validating” to render the regulations as lawful. I argue that these regulatory enactments materialized estrogen as a potent, unpredictable, and multiple object. Further, I show how estrogen spawned novel regulatory techniques in Canada, particularly the use of consumer product labels. In this way, estrogen catalyzed an early example of risk regulation in Canada. -

Increased and Mistimed Sex Hormone Production in Night Shift Workers
Published OnlineFirst March 3, 2015; DOI: 10.1158/1055-9965.EPI-14-1271 Research Article Cancer Epidemiology, Biomarkers Increased and Mistimed Sex Hormone Production & Prevention in Night Shift Workers Kyriaki Papantoniou1,2,3,4, Oscar J. Pozo2, Ana Espinosa1,2,3,4, Josep Marcos2,3, Gemma Castano-Vinyals~ 1,2,3,4, Xavier Basagana~ 1,2,3,4, Elena Juanola Pages 5, Joan Mirabent6,7, Jordi Martín8, Patricia Such Faro9, Amparo Gasco Aparici10, Benita Middleton11, Debra J. Skene11, and Manolis Kogevinas1,2,3,4,12 Abstract Background: Night shift work has been associated with Results: Night workers had higher levels of total progestagens an increased risk for breast and prostate cancer. The effect [geometric mean ratio (GMR) 1.65; 95% confidence intervals of circadian disruption on sex steroid production is a pos- (CI), 1.17–2.32] and androgens (GMR: 1.44; 95% CI, 1.03–2.00), sible underlying mechanism, underinvestigated in hum- compared with day workers, after adjusting for potential con- ans. We have assessed daily rhythms of sex hormones founders. The increased sex hormone levels among night and melatonin in night and day shift workers of both shift workers were not related to the observed suppression of sexes. 6-sulfatoxymelatonin. Peak time of androgens was significantly Methods: We recruited 75 night and 42 day workers, ages later among night workers, compared with day workers (testos- 22 to 64 years, in different working settings. Participants terone: 12:14 hours; 10:06-14:48 vs. 08:35 hours; 06:52-10:46). collected urine samples from all voids over 24 hours on a Conclusions: We found increased levels of progestagens and working day. -

1970Qureshiocr.Pdf (10.44Mb)
STUDY INVOLVING METABOLISM OF 17-KETOSTEROIDS AND 17-HYDROXYCORTICOSTEROIDS OF HEALTHY YOUNG MEN DURING AMBULATION AND RECUMBENCY A DISSERTATION SUBMITTED IN PARTIAL FULFILLMENT OF THE REQUIREMENTS FOR THE DEGREE OF DOCTOR OF PHILOSOPHY IN NUTRITION IN THE GRADUATE DIVISION OF THE TEXAS WOI\IIAN 'S UNIVERSITY COLLEGE OF HOUSEHOLD ARTS AND SCIENCES BY SANOBER QURESHI I B .Sc. I M.S. DENTON I TEXAS MAY I 1970 ACKNOWLEDGMENTS The author wishes to express her sincere gratitude to those who assisted her with her research problem and with the preparation of this dissertation. To Dr. Pauline Beery Mack, Director of the Texas Woman's University Research Institute, for her invaluable assistance and gui dance during the author's entire graduate program, and for help in the preparation of this dissertation; To the National Aeronautics and Space Administration for their support of the research project of which the author's study is a part; To Dr. Elsa A. Dozier for directing the author's s tucly during 1969, and to Dr. Kathryn Montgomery beginning in early 1970, for serving as the immeclia te director of the author while she was working on the completion of the investic;ation and the preparation of this dis- sertation; To Dr. Jessie Bateman, Dean of the College of Household Arts and Sciences, for her assistance in all aspects of the author's graduate program; iii To Dr. Ralph Pyke and Mr. Walter Gilchrist 1 for their ass is tance and generous kindness while the author's research program was in progress; To Mr. Eugene Van Hooser 1 for help during various parts of her research program; To Dr.