Life History Patterns and the Comparative Social Ecology of Carnivores
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
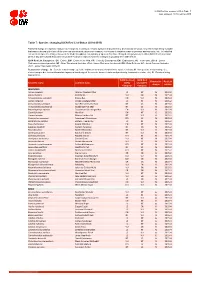
Table 7: Species Changing IUCN Red List Status (2014-2015)
IUCN Red List version 2015.4: Table 7 Last Updated: 19 November 2015 Table 7: Species changing IUCN Red List Status (2014-2015) Published listings of a species' status may change for a variety of reasons (genuine improvement or deterioration in status; new information being available that was not known at the time of the previous assessment; taxonomic changes; corrections to mistakes made in previous assessments, etc. To help Red List users interpret the changes between the Red List updates, a summary of species that have changed category between 2014 (IUCN Red List version 2014.3) and 2015 (IUCN Red List version 2015-4) and the reasons for these changes is provided in the table below. IUCN Red List Categories: EX - Extinct, EW - Extinct in the Wild, CR - Critically Endangered, EN - Endangered, VU - Vulnerable, LR/cd - Lower Risk/conservation dependent, NT - Near Threatened (includes LR/nt - Lower Risk/near threatened), DD - Data Deficient, LC - Least Concern (includes LR/lc - Lower Risk, least concern). Reasons for change: G - Genuine status change (genuine improvement or deterioration in the species' status); N - Non-genuine status change (i.e., status changes due to new information, improved knowledge of the criteria, incorrect data used previously, taxonomic revision, etc.); E - Previous listing was an Error. IUCN Red List IUCN Red Reason for Red List Scientific name Common name (2014) List (2015) change version Category Category MAMMALS Aonyx capensis African Clawless Otter LC NT N 2015-2 Ailurus fulgens Red Panda VU EN N 2015-4 -

Classification of Mammals 61
© Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FORCHAPTER SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION Classification © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC 4 NOT FORof SALE MammalsOR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC © Jones & Bartlett Learning, LLC NOT FOR SALE OR DISTRIBUTION NOT FOR SALE OR DISTRIBUTION © Jones & Bartlett Learning, LLC. NOT FOR SALE OR DISTRIBUTION. 2ND PAGES 9781284032093_CH04_0060.indd 60 8/28/13 12:08 PM CHAPTER 4: Classification of Mammals 61 © Jones Despite& Bartlett their Learning,remarkable success, LLC mammals are much less© Jones stress & onBartlett the taxonomic Learning, aspect LLCof mammalogy, but rather as diverse than are most invertebrate groups. This is probably an attempt to provide students with sufficient information NOT FOR SALE OR DISTRIBUTION NOT FORattributable SALE OR to theirDISTRIBUTION far greater individual size, to the high on the various kinds of mammals to make the subsequent energy requirements of endothermy, and thus to the inabil- discussions of mammalian biology meaningful. -

Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes Macrotis)
Author's Personal Copy J. Comp. Path. 2019, Vol. 167, 60e72 Available online at www.sciencedirect.com ScienceDirect www.elsevier.com/locate/jcpa DISEASE IN WILDLIFE OR EXOTIC SPECIES Dental and Temporomandibular Joint Pathology of the Kit Fox (Vulpes macrotis) N. Yanagisawa*, R. E. Wilson*, P. H. Kass† and F. J. M. Verstraete* *Department of Surgical and Radiological Sciences and † Department of Population Health and Reproduction, School of Veterinary Medicine, University of California, Davis, California, USA Summary Skull specimens from 836 kit foxes (Vulpes macrotis) were examined macroscopically according to predefined criteria; 559 specimens were included in this study. The study group consisted of 248 (44.4%) females, 267 (47.8%) males and 44 (7.9%) specimens of unknown sex; 128 (22.9%) skulls were from young adults and 431 (77.1%) were from adults. Of the 23,478 possible teeth, 21,883 teeth (93.2%) were present for examina- tion, 45 (1.9%) were absent congenitally, 405 (1.7%) were acquired losses and 1,145 (4.9%) were missing ar- tefactually. No persistent deciduous teeth were observed. Eight (0.04%) supernumerary teeth were found in seven (1.3%) specimens and 13 (0.06%) teeth from 12 (2.1%) specimens were malformed. Root number vari- ation was present in 20.3% (403/1,984) of the present maxillary and mandibular first premolar teeth. Eleven (2.0%) foxes had lesions consistent with enamel hypoplasia and 77 (13.8%) had fenestrations in the maxillary alveolar bone. Periodontitis and attrition/abrasion affected the majority of foxes (71.6% and 90.5%, respec- tively). -

Helogale Parvula)
Vocal Recruitment in Dwarf Mongooses (Helogale parvula) Janneke Rubow Thesis presented in fulfilment of the requirements for the degree of Master of Science in the Faculty of Science at Stellenbosch University Supervisor: Prof. Michael I. Cherry Co-supervisor: Dr. Lynda L. Sharpe March 2017 Stellenbosch University https://scholar.sun.ac.za DECLARATION By submitting this thesis electronically, I declare that the entirety of the work contained therein is my own, original work, that I am the sole author thereof (save to the extent explicitly otherwise stated), that reproduction and publication thereof by Stellenbosch University will not infringe any third party rights and that I have not previously in its entirety or in part submitted it for obtaining any qualification. Janneke Rubow, March 2017 Copyright © 2017 Stellenbosch University All rights reserved Stellenbosch University https://scholar.sun.ac.za Abstract Vocal communication is important in social vertebrates, particularly those for whom dense vegetation obscures visual signals. Vocal signals often convey secondary information to facilitate rapid and appropriate responses. This function is vital in long-distance communication. The long-distance recruitment vocalisations of dwarf mongooses (Helogale parvula) provide an ideal opportunity to study informative cues in acoustic communication. This study examined the information conveyed by two recruitment calls given in snake encounter and isolation contexts, and whether dwarf mongooses are able to respond differently on the basis of these cues. Vocalisations were collected opportunistically from four wild groups of dwarf mongooses. The acoustic parameters of recruitment calls were then analysed for distinction between contexts within recruitment calls in general, distinction within isolation calls between groups, sexes and individuals, and the individuality of recruitment calls in comparison to dwarf mongoose contact calls. -

EXTRA-PAIR MATING and EFFECTIVE POPULATION SIZE in the SONG SPARROW (Melospiza Melodia)
EXTRA-PAIR MATING AND EFFECTIVE POPULATION SIZE IN THE SONG SPARROW (Melospiza melodia) By Kathleen D. O'Connor B.A., Skidmore College, 2000 A THESIS SUBMITTED IN PARTIAL FUFILMENT OF THE REQUIREMENTS FOR THE DEGREE OF MASTER OF SCIENCE in THE FACULTY OF GRADUATE STUDIES THE FACULTY OF FORESTRY Department of Forest Sciences Centre for Applied Conservation Research We accept this thesis as conforming to the required standard THE UNIVERSITY OF BRITISH COLUMBIA April 2003 © Kathleen D. O'Connor, 2003 In presenting this thesis in partial fulfilment of the requirements for an advanced degree at the University of British Columbia, I agree that the Library shall make it freely available for reference and study. I further agree that permission for extensive copying of this thesis for scholarly purposes may be granted by the head of my department or by his or her representatives. It is understood that copying or publication of this thesis for financial gain shall not be allowed without my written permission. Department of Fpce_t Sciences The University of British Columbia Vancouver, Canada Date 2HApc. 20O5 DE-6 (2/88) Abstract Effective population size is used widely in conservation research and management as an indicator of the genetic state of populations. However, estimates of effective population size for socially monogamous species can vary with the frequency of matings outside of the social pair. I investigated the effect of cryptic extra-pair fertilization on effective population size estimates using four years of demographic and genetic data from a resident population of song sparrows (Melospiza melodia Oberholser 1899) on Mandarte Island, British Columbia, Canada. -

Resource Partitioning Between Black-Backed Jackal and Brown Hyaena in Waterberg Biosphere Reserve, South Africa
Ramnanan et al. Jackal and hyaena in South Africa Copyright © 2016 by the IUCN/SSC Canid Specialist Group. ISSN 1478-2677 Research report Resource partitioning between black-backed jackal and brown hyaena in Waterberg Biosphere Reserve, South Africa Rivona Ramnanan1, Michelle Thorn1, Craig J. Tambling2 and Michael J. Somers 1, 3* 1 Centre for Wildlife Management, University of Pretoria, Pretoria, South Africa. 2 Centre for African Conservation Ecology, Department of Zoology, Nelson Mandela Metropolitan University, Port Elizabeth, 6031, South Africa. 3 Centre for Invasion Biology, University of Pretoria, Pretoria, South Africa. Email: [email protected] * Correspondence author Keywords: apex predators, dietary niche, human modification, resource partitioning. Abstract Understanding resource partitioning by predators is important for understanding coexistence patterns, with this becoming more relevant as historical food webs are altered through human impacts. Using scat analysis, we investigated the diet overlap of two sympatric meso-carnviores, the black-backed jackal Canis mesomelas and brown hyaena Hyaena brunnea, in Waterberg Biosphere Reserve, South Africa. Scats (n = 30 jackal, 42 brown hyaena) were collected in April 2012 from game and livestock farms. When comparing main prey categories (medium-large mam- mal, small mammal, fruit, invertebrate, reptile, and bird) we found little difference in diets, with both carnivores con- suming predominantly medium-large mammals (10-100kg). Bushbuck Tragelaphus scriptus was the most commonly consumed large mammal species for both predators. Jackal and brown hyaena had, on average, 1.3 and 1.4 main prey categories per scat respectively which resulted in diet diversities of 3.9 for jackal and 2.5 for brown hyaena. -

Foraging : an Ecology Model of Consumer Behaviour?
This is a repository copy of Foraging : an ecology model of consumer behaviour?. White Rose Research Online URL for this paper: https://eprints.whiterose.ac.uk/122432/ Version: Accepted Version Article: Wells, VK orcid.org/0000-0003-1253-7297 (2012) Foraging : an ecology model of consumer behaviour? Marketing Theory. pp. 117-136. ISSN 1741-301X https://doi.org/10.1177/1470593112441562 Reuse Items deposited in White Rose Research Online are protected by copyright, with all rights reserved unless indicated otherwise. They may be downloaded and/or printed for private study, or other acts as permitted by national copyright laws. The publisher or other rights holders may allow further reproduction and re-use of the full text version. This is indicated by the licence information on the White Rose Research Online record for the item. Takedown If you consider content in White Rose Research Online to be in breach of UK law, please notify us by emailing [email protected] including the URL of the record and the reason for the withdrawal request. [email protected] https://eprints.whiterose.ac.uk/ Foraging: an ecology model of consumer behavior? Victoria.K.Wells* Durham Business School, UK First Submission June 2010 Revision One December 2010 Revision Two August 2011 Accepted for Publication: September 2011 * Address for correspondence: Dr Victoria Wells (née James), Durham Business School, Durham University, Mill Hill Lane, Durham, DH1 3LB & Queen’s Campus, University Boulevard, Thornaby, Stockton-on-Tees, TS17 6BH, Telephone: +44 (0)191 334 0472, E-mail: [email protected] I would like to thank Tony Ellson for his guidance and Gordon Foxall for his helpful comments during the development and writing of this paper. -

3.4 ORDER CARNIVORA Bowdich, 1821
3.4 ORDER CARNIVORA Bowdich, 1821 3.4.1 Family Ursidae Fischer, 1817 There are eight species of bears in the world: - American Black Bear Ursus americanus - Brown Bear Ursus arctos - Polar Bear Ursus maritimus - Sloth Bear Melursus ursinus - Spectacled Bear Tremarctos ornatus - Giant Panda Ailuropoda melanoleuca - Asiatic Black Bear Ursus thibetanus - Malayan Sun Bear Helarctos malayanus The last two species are the only members of the family Ursidae known in Southeast Asia. They differ from each other by their furs and body sizes and both are threatened with extinction (Nowak, 1991; Corbet & Hill 1992). Bears have relatively undeveloped carnassial teeth; narrow premolars, crushing molars with flat crowns and large robust canines. 127 3.4.1.1 Subfamily Ursinae Fischer, 1817, Plate 3(A1 to B3) As mentioned above, two genera and two species represent the subfamily Ursinae in Southeast Asia, namely: - Malayan Sun Bear (Figure 3.8, A), Ursus/Helarctos malayanus (Raffles, 1821) with the scientific name Ursu and synonym Helarctos is distributed in the south west of China, Assam, Myanmar, Vietnam, Peninsular Malaysia, to the islands of Sumatra and Borneo. It is the smallest of all bears found in the tropical rainforests of Southeast Asia. - Asiatic Black Bear (Figure 3.8, B), Ursus thibetanus Cuvier, 1823 is mainly localized in the Himalayas, Afghanistan to southern China, Myanmar, northern Thailand and Indochina. It has several alternative names including Asiatic Black Bear, Himalayan Black Bear, Moon Bear and inhabits mountain forests. Figure 3.8 Malayan Sun Bear (A) and Asiatic Black Bear (B) in Zoo Negara, Malaysia National Zoological Park. -

Controlled Animals
Environment and Sustainable Resource Development Fish and Wildlife Policy Division Controlled Animals Wildlife Regulation, Schedule 5, Part 1-4: Controlled Animals Subject to the Wildlife Act, a person must not be in possession of a wildlife or controlled animal unless authorized by a permit to do so, the animal was lawfully acquired, was lawfully exported from a jurisdiction outside of Alberta and was lawfully imported into Alberta. NOTES: 1 Animals listed in this Schedule, as a general rule, are described in the left hand column by reference to common or descriptive names and in the right hand column by reference to scientific names. But, in the event of any conflict as to the kind of animals that are listed, a scientific name in the right hand column prevails over the corresponding common or descriptive name in the left hand column. 2 Also included in this Schedule is any animal that is the hybrid offspring resulting from the crossing, whether before or after the commencement of this Schedule, of 2 animals at least one of which is or was an animal of a kind that is a controlled animal by virtue of this Schedule. 3 This Schedule excludes all wildlife animals, and therefore if a wildlife animal would, but for this Note, be included in this Schedule, it is hereby excluded from being a controlled animal. Part 1 Mammals (Class Mammalia) 1. AMERICAN OPOSSUMS (Family Didelphidae) Virginia Opossum Didelphis virginiana 2. SHREWS (Family Soricidae) Long-tailed Shrews Genus Sorex Arboreal Brown-toothed Shrew Episoriculus macrurus North American Least Shrew Cryptotis parva Old World Water Shrews Genus Neomys Ussuri White-toothed Shrew Crocidura lasiura Greater White-toothed Shrew Crocidura russula Siberian Shrew Crocidura sibirica Piebald Shrew Diplomesodon pulchellum 3. -

Canid, Hye A, Aardwolf Conservation Assessment and Management Plan (Camp) Canid, Hyena, & Aardwolf
CANID, HYE A, AARDWOLF CONSERVATION ASSESSMENT AND MANAGEMENT PLAN (CAMP) CANID, HYENA, & AARDWOLF CONSERVATION ASSESSMENT AND MANAGEMENT PLAN (CAMP) Final Draft Report Edited by Jack Grisham, Alan West, Onnie Byers and Ulysses Seal ~ Canid Specialist Group EARlliPROMSE FOSSIL RIM A fi>MlY Of CCNSERVA11QN FUNDS A Joint Endeavor of AAZPA IUCN/SSC Canid Specialist Group IUCN/SSC Hyaena Specialist Group IUCN/SSC Captive Breeding Specialist Group CBSG SPECIES SURVIVAL COMMISSION The work of the Captive Breeding Specialist Group is made possible by gellerous colltributiolls from the following members of the CBSG Institutional Conservation Council: Conservators ($10,000 and above) Federation of Zoological Gardens of Arizona-Sonora Desert Museum Claws 'n Paws Australasian Species Management Program Great Britain and Ireland BanhamZoo Darmstadt Zoo Chicago Zoological Society Fort Wayne Zoological Society Copenhagen Zoo Dreher Park Zoo Columbus Zoological Gardens Gladys Porter Zoo Cotswold Wildlife Park Fota Wildlife Park Denver Zoological Gardens Indianapolis Zoological Society Dutch Federation of Zoological Gardens Great Plains Zoo Fossil Rim Wildlife Center Japanese Association of Zoological Parks Erie Zoological Park Hancock House Publisher Friends of Zoo Atlanta and Aquariums Fota Wildlife Park Kew Royal Botanic Gardens Greater Los Angeles Zoo Association Jersey Wildlife Preservation Trust Givskud Zoo Miller Park Zoo International Union of Directors of Lincoln Park Zoo Granby Zoological Society Nagoya Aquarium Zoological Gardens The Living Desert Knoxville Zoo National Audubon Society-Research Metropolitan Toronto Zoo Marwell Zoological Park National Geographic Magazine Ranch Sanctuary Minnesota Zoological Garden Milwaukee County Zoo National Zoological Gardens National Aviary in Pittsburgh New York Zoological Society NOAHS Center of South Africa Parco Faunistico "La To:rbiera" Omaha's Henry Doorly Zoo North of Chester Zoological Society Odense Zoo Potter Park Zoo Saint Louis Zoo Oklahoma City Zoo Orana Park Wildlife Trust Racine Zoological Society Sea World, Inc. -

Mammalian Predators Appropriating the Refugia of Their Prey
Mamm Res (2015) 60:285–292 DOI 10.1007/s13364-015-0236-y ORIGINAL PAPER When prey provide more than food: mammalian predators appropriating the refugia of their prey William J. Zielinski 1 Received: 30 September 2014 /Accepted: 20 July 2015 /Published online: 31 July 2015 # Mammal Research Institute, Polish Academy of Sciences, Białowieża, Poland (outside the USA) 2015 Abstract Some mammalian predators acquire both food and predators) may play disproportionately important roles in their shelter from their prey, by eating them and using the refugia communities. the prey construct. I searched the literature for examples of predators that exhibit this behavior and summarize their taxo- Keywords Predator–prey . Dens . Herbivore . Behavior . nomic affiliations, relative sizes, and distributions. I hypothe- Habitat . Resting . Foraging sized that size ratios of species involved in this dynamic would be near 1.0, and that most of these interactions would occur at intermediate and high latitudes. Seventeen species of Introduction Carnivorans exploited at least 23 species of herbivores as food and for their refugia. Most of them (76.4 %) were in the Mammals require food and most require shelter, either to pro- Mustelidae; several small species of canids and a few tect them from predators or from thermal stress. Carnivorous herpestids were exceptions. Surprisingly, the average mammals are unique in that they subsist on mobile food predator/prey weight ratio was 10.51, but few species of pred- sources which, particularly if these sources are vertebrates, ators were more than ten times the weight of the prey whose may build their own refuges to help regulate their body tem- refugia they exploit. -

Cranial Morphological Distinctiveness Between Ursus Arctos and U
East Tennessee State University Digital Commons @ East Tennessee State University Electronic Theses and Dissertations Student Works 5-2017 Cranial Morphological Distinctiveness Between Ursus arctos and U. americanus Benjamin James Hillesheim East Tennessee State University Follow this and additional works at: https://dc.etsu.edu/etd Part of the Biodiversity Commons, Evolution Commons, and the Paleontology Commons Recommended Citation Hillesheim, Benjamin James, "Cranial Morphological Distinctiveness Between Ursus arctos and U. americanus" (2017). Electronic Theses and Dissertations. Paper 3261. https://dc.etsu.edu/etd/3261 This Thesis - Open Access is brought to you for free and open access by the Student Works at Digital Commons @ East Tennessee State University. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons @ East Tennessee State University. For more information, please contact [email protected]. Cranial Morphological Distinctiveness Between Ursus arctos and U. americanus ____________________________________ A thesis presented to the Department of Geosciences East Tennessee State University In partial fulfillment of the requirements for the degree Master of Science in Geosciences ____________________________________ by Benjamin Hillesheim May 2017 ____________________________________ Dr. Blaine W. Schubert, Chair Dr. Steven C. Wallace Dr. Josh X. Samuels Keywords: Ursidae, Geometric morphometrics, Ursus americanus, Ursus arctos, Last Glacial Maximum ABSTRACT Cranial Morphological Distinctiveness Between Ursus arctos and U. americanus by Benjamin J. Hillesheim Despite being separated by millions of years of evolution, black bears (Ursus americanus) and brown bears (Ursus arctos) can be difficult to distinguish based on skeletal and dental material alone. Complicating matters, some Late Pleistocene U. americanus are significantly larger in size than their modern relatives, obscuring the identification of the two bears.