J Clin Densitom
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-
(12) United States Patent (10) Patent No.: US 7,074,961 B2 Wang Et Al
US007074961B2 (12) United States Patent (10) Patent No.: US 7,074,961 B2 Wang et al. (45) Date of Patent: Jul. 11, 2006 (54) ANTIDEPRESSANTS AND THEIR 6.211,171 B1 4/2001 Sawynok et al. ANALOGUES AS LONG-ACTING LOCAL 6,545,057 B1 4/2003 Wang et al. ANESTHETCS AND ANALGESCS 2001/0036943 A1 11/2001 Coe et al. (75) Inventors: Ging Kuo Wang, Westwood, MA (US); FOREIGN PATENT DOCUMENTS Peter Gerner, Weston, MA (US); Donald K. Verrecchia, Winchester, MA CH 534124 A 2, 1973 (US) WO WO95/17903 A1 7, 1995 WO WO95/1818.6 A1 7, 1995 (73) Assignee: The Brigham and Women's Hospital, WO WO 99.59.598 A1 11, 1999 Inc., Boston, MA (US) WO WO O2/060870 A2 8, 2002 (*) Notice: Subject to any disclaimer, the term of this OTHER PUBLICATIONS patent is extended or adjusted under 35 U.S.C. 154(b) by 451 days. Luo et al., Xenobiotica, vol. 25, No. 3, pp. 291-301, 1995.* (21) Appl. No.: 10/117,708 Ehlert et al., The Interaction of Amitriptyline, Doxepin, Imipramine and Their N-Methyl Quaternary Ammonium (22) Filed: Apr. 4, 2002 Derivatives with Subtypes of Muscarinic Receptors in Brain (65) Prior Publication Data and Heart, The Journal of Pharmacology and Experimental Therapeutics, Apr. 1990, vol. 253, No. 1, pp. 13–19. US 2003/00968.05 A1 May 22, 2003 Gerner, P., et al., Anesthesiology (2002) 96:1435–42. Related U.S. Application Dat e pplication Uata Khan, M.A., et al., Anesthesiology (2002) 96:109–16. (63) stripps'965,138, filed on Sep. -

Curriculum Vitae: Daniel J
CURRICULUM VITAE: DANIEL J. WALLACE, M.D., F.A.C.P., M.A.C.R. Up to date as of January 1, 2019 Personal: Address: 8750 Wilshire Blvd, Suite 350 Beverly Hills, CA 90211 Phone: (310) 652-0010 FAX: (310) 360-6219 E mail: [email protected] Education: University of Southern California, 2/67-6/70, BA Medicine, 1971. University of Southern California, 9/70-6/74, M.D, 1974. Postgraduate Training: 7/74-6/75 Medical Intern, Rhode Island (Brown University) Hospital, Providence, RI. 7/75-6/77 Medical Resident, Cedars-Sinai Medical Center, Los Angeles, CA. 7/77-6/79 Rheumatology Fellow, UCLA School of Medicine, Los Angeles, CA. Medical Boards and Licensure: Diplomate, National Board of Medical Examiners, 1975. Board Certified, American Board of Internal Medicine, 1978. Board Certified, Rheumatology subspecialty, 1982. California License: #G-30533. Present Appointments: Medical Director, Wallace Rheumatic Study Center Attune Health Affiliate, Beverly Hills, CA 90211 Attending Physician, Cedars-Sinai Medical Center, Los Angeles, 1979- Clinical Professor of Medicine, David Geffen School of Medicine at UCLA, 1995- Professor of Medicine, Cedars-Sinai Medical Center, 2012- Expert Reviewer, Medical Board of California, 2007- Associate Director, Rheumatology Fellowship Program, Cedars-Sinai Medical Center, 2010- Board of Governors, Cedars-Sinai Medical Center, 2016- Member, Medical Policy Committee, United Rheumatology, 2017- Honorary Appointments: Fellow, American College of Physicians (FACP) Fellow, American College of Rheumatology (FACR) -

Testosterone, Myocardial Function, and Mortality
Heart Failure Reviews https://doi.org/10.1007/s10741-018-9721-0 Testosterone, myocardial function, and mortality Vittorio Emanuele Bianchi1 # Springer Science+Business Media, LLC, part of Springer Nature 2018 Abstract The cardiovascular system is particularly sensitive to androgens, but some controversies exist regarding the effect of testosterone on the heart. While among anabolic abusers, cases of sudden cardiac death have been described, recently it was reported that low serum level of testosterone was correlated with increased risk of cardiovascular diseases (CVD) and mortality rate. This review aims to evaluate the effect of testosterone on myocardial tissue function, coronary artery disease (CAD), and death. Low testosterone level is associated with increased incidence of CAD and mortality. Testosterone administration in hypogonadal elderly men and women has a positive effect on cardiovascular function and improved clinical outcomes and survival time. Although at supraphysiologic doses, androgen may have a toxic effect, and at physiological levels, testosterone is safe and exerts a beneficial effect on myocardial function including mechanisms at cellular and mitochondrial level. The interaction with free testosterone and estradiol should be considered. Further studies are necessary to better understand the interaction mechanisms for an optimal androgen therapy in CVD. Keywords Testosterone . Coronary artery disease . Heart failure . Cardiovascular disease . Cardiomyocytes . Cardiac mitochondria . Cardiovascular mortality Introduction essential role [20]. Asymptomatic hypogonadism is observed in men 30–79 years old with an incidence of about 5.6% [21] The effects of testosterone on myocardial function have gen- and is more relevant in aging subjects. In recent years, pre- erated a great deal of discussion in the literature but remain scriptions for testosterone replacement therapy have increased controversial. -

BDNF Genotype Modulates Resting Functional Connectivity in Children
ORIGINAL RESEARCH ARTICLE published: 24 November 2009 HUMAN NEUROSCIENCE doi: 10.3389/neuro.09.055.2009 BDNF genotype modulates resting functional connectivity in children Moriah E. Thomason1*, Daniel J. Yoo1, Gary H. Glover 2 and Ian H. Gotlib1 1 Department of Psychology, Stanford University, Stanford, CA, USA 2 Department of Radiology, Stanford University School of Medicine, Stanford, CA, USA Edited by: A specifi c polymorphism of the brain-derived neurotrophic factor (BDNF) gene is associated Elizabeth D. O’Hare, University of with alterations in brain anatomy and memory; its relevance to the functional connectivity of California at Berkeley, USA brain networks, however, is unclear. Given that altered hippocampal function and structure Reviewed by: Damien Fair, Oregon Health and has been found in adults who carry the methionine (met) allele of the BDNF gene and the Science University, USA molecular studies elucidating the role of BDNF in neurogenesis and synapse formation, we Naftali Raz, Wayne State University, examined the association between BDNF gene variants and neural resting connectivity in USA children and adolescents. We observed a reduction in hippocampal and parahippocampal to *Correspondence: cortical connectivity in met-allele carriers within both default-mode and executive networks. Moriah E. Thomason, Department of Psychology, Stanford University, Jordan In contrast, we observed increased connectivity to amygdala, insula and striatal regions in Hall, Bldg. 420, Stanford, met-carriers, within the paralimbic network. Because of the known association between the CA 94305-2130, USA. BDNF gene and neuropsychiatric disorder, this latter fi nding of greater connectivity in circuits e-mail: [email protected] important for emotion processing may indicate a new neural mechanism through which these gene-related psychiatric differences are manifest. -

Phase II Study of Dehydroepiandrosterone in Androgen Receptor-Positive Metastatic Breast Cancer
Published Ahead of Print on December 27, 2018 as 10.1634/theoncologist.2018-0243. Clinical Trial Results Phase II Study of Dehydroepiandrosterone in Androgen Receptor-Positive Metastatic Breast Cancer a b c c c b ELISABETTA PIETRI, ILARIA MASSA, SARA BRAVACCINI, SARA RAVAIOLI, MARIA MADDALENA TUMEDEI, ELISABETTA PETRACCI, d a e f g h i CATERINA DONATI, ALESSIO SCHIRONE, FEDERICO PIACENTINI, LORENZO GIANNI, MARIO NICOLINI, ENRICO CAMPADELLI, ALESSANDRA GENNARI, j j b b c a a ALESSANDRO SABA, BEATRICE CAMPI, LINDA VALMORRI, DANIELE ANDREIS, FRANCESCO FABBRI, DINO AMADORI, ANDREA ROCCA aDepartment of Medical Oncology, bUnit of Biostatistics and Clinical Trials, cBiosciences Laboratory, and dOncology Pharmacy Unit, Istituto Scientifico Romagnolo per lo Studio e la Cura dei Tumori (IRST) IRCCS, Meldola, Italy; eDivision of Medical Oncology, Department of Medical and Surgical Sciences for Children & Adults, University Hospital of Modena, Modena, Italy; fDepartment of Oncology, Infermi Hospital, Rimini, Italy; gOncology Day Hospital Unit, Cervesi Hospital, Cattolica, Italy; hOncology Unit, Faenza Hospital, Faenza, Italy; iMedical Oncology, Department of Translational Medicine, University of Eastern Piedmont, Novara, Italy; jDepartment of Surgical, Medical and Molecular Pathology and Critical Care Medicine, University of Pisa, Pisa, Italy Downloaded from TRIAL INFORMATION http://theoncologist.alphamedpress.org/ • ClinicalTrials.gov Identifier: NCT02000375 • Principal Investigator: Elisabetta Pietri • Sponsor(s): None • IRB Approved: Yes LESSONS LEARNED • The androgen receptor (AR) is present in most breast cancers (BC), but its exploitation as a therapeutic target has been limited. • This study explored the activity of dehydroepiandrosterone (DHEA), a precursor being transformed into androgens within BC cells, in combination with an aromatase inhibitor (to block DHEA conversion into estrogens), in a two-stage phase II study in patients with AR-positive/estrogen receptor-positive/human epidermal growth receptor 2-negative metastatic BC. -

The Role of the Small Gtpase Rap in Drosophila R7 Photoreceptor Specification
The role of the small GTPase Rap in Drosophila R7 photoreceptor specification Yannis Emmanuel Mavromatakis and Andrew Tomlinson1 Department of Genetics and Development, College of Physicians and Surgeons, Columbia University, New York, NY 10032 Edited* by Richard Axel, Columbia University, New York, NY, and approved January 25, 2012 (received for review September 13, 2011) The Drosophila R7 photoreceptor provides an excellent model sys- and R7 precursors differ dramatically in their levels of RTK tem with which to study how cells receive and “decode” signals signaling: it is mild in the former and robust in the latter. that specify cell fate. R7 is specified by the combined actions of the RTK signaling typically activates the small GTPase Ras, which receptor tyrosine kinase (RTK) and Notch (N) signaling pathways. recruits Raf to the plasma membrane and initiates the phos- These pathways interact in a complex manner that includes antag- phorylation cascade that leads to the translocation of MAPK to onistic effects on photoreceptor specification: RTK promotes the the nucleus. Rap is another member of the Ras GTPase super- photoreceptor fate, whereas N inhibits. Although other photore- family; it was initially considered a Ras antagonist because it sup- ceptors are subject to only mild N activation, R7 experiences a high- pressed Ki-Ras oncogenesis (17) and inhibited Ras-dependent level N signal. To counter this effect and to ensure that the cell is activation of MAPK (18). Roughened is a dominant mutant of specified as a photoreceptor, a high RTK signal is transduced in the Drosophila rap in which R7 photoreceptors are frequently absent cell. -

Q3D(R1) Elemental Impurities
Q3D(R1) Elemental Impurities Guidance for Industry U. S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) March 2020 ICH Revision 1 Q3D(R1) Elemental Impurities Guidance for Industry Additional copies are available from: Office of Communications, Division of Drug Information Center for Drug Evaluation and Research Food and Drug Administration 10001 New Hampshire Ave., Hillandale Bldg., 4th Floor Silver Spring, MD 20993-0002 Phone: 855-543-3784 or 301-796-3400; Fax: 301-431-6353 Email: [email protected] https://www.fda.gov/drugs/guidance-compliance-regulatory-information/guidances-drugs and/or Office of Communication, Outreach and Development Center for Biologics Evaluation and Research Food and Drug Administration 10903 New Hampshire Ave., Bldg. 71, Room 3128 Silver Spring, MD 20993-0002 Phone: 800-835-4709 or 240-402-8010 Email : [email protected] https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) March 2020 ICH Revision 1 Contains Nonbinding Recommendations TABLE OF CONTENTS I. INTRODUCTION (1) ....................................................................................................... 1 II. SCOPE (2)......................................................................................................................... -
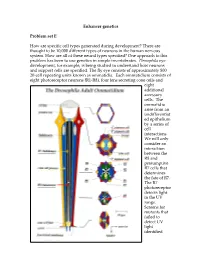
Enhancer Genetics Problem Set E How Are
Enhancer genetics Problem set E How are specific cell types generated during development? There are thought to be 10,000 different types of neurons in the human nervous system. How are all of these neural types specified? One approach to this problem has been to use genetics in simple invertebrates. Drosophila eye development, for example, is being studied to understand how neurons and support cells are specified. The fly eye consists of approximately 800 20-cell repeating units known as ommatidia. Each ommatidium consists of eight photoreceptor neurons (R1-R8), four lens secreting cone cells and eight additional accessory cells. The ommatidia arise from an undifferentiat ed epithelium by a series of cell interactions. We will only consider an interaction between the R8 and presumptive R7 cells that determines the fate of R7. The R7 photoreceptor detects light in the UV range. Screens for mutants that failed to detect UV light identified genes that were necessary for formation of R7 cells: mutants failed to detect UV because they had ommatidia that lacked R7 cells. Two of the genes were sevenless (sev) and bride of sevenless (Boss). Adult flies homozygous for mutations in these genes have ommatidia that lack an R7 cell and contain an additional cone cell. In the absence of R7 differentiation, the presumptive R7 cell becomes a cone cell. sev is a receptor tyrosine kinase, and it acts in R7 to specify R7's fate. boss encodes the ligand for the Sev receptor tyrosine kinase, and in contrast to sev, acts in R8 cell to specify R7's fate. -

Potentiation of Neuronal NMDA Response Induced by Dehydroepiandrosterone and Its Suppression by Progesterone: Effects Mediated Via Sigma Receptors
The Journal of Neuroscience, February 1, 1996, 76(3):1193-l 202 Potentiation of Neuronal NMDA Response Induced by Dehydroepiandrosterone and its Suppression by Progesterone: Effects Mediated via Sigma Receptors Richard Bergeron, Claude de Montigny, and Guy Debonnel Department of Psychiatry, Neurobiological Psychiatry Unit, McGill University, Montreal, Quebec, Canada H3A 1 A 1 We have shown previously that low doses of selective sigma well as those induced by nonsteroidal u ligands. Neither preg- (cr)-receptor ligands potentiate the excitatory response of py- nenolone nor pregnenolone sulfate had any effect on the NMDA ramidal neurons to NMDA in the CA, region of the dorsal response-nor did they antagonize the potentiation of the hippocampus in the rat. Because progesterone competitively NMDA response induced by DHEA and by nonsteroidal IJ displaces the binding of the ligand N-[3H]allyl-normetazocine ligands. A pet-tussis toxin pretreatment, which inactivates G,,,- (SKF-10,047), the present studies were undertaken to deter- proteins, abolished the potentiating effects of DHEA. Ovariec- mine in viva the effect of neuroactive steroids on NMDA- tomy enhanced the potentiation of the NMDA response by the induced excitation of rat CA, pyramidal neurons. Low doses of nonsteroidal CT ligand di(2-tolyl)guanidine (DTG). There was a dehydroepiandrosterone (DHEA) potentiated the NMDA re- reciprocal occlusion of the effects of DHEA and DTG; DTG did sponse selectively and dose-dependently. The effect of DHEA not potentiate the NMDA response further after DHEA, and was reversed by the selective v antagonist N-dipropyl-2-(4- DHEA did not do so after DTG. These results suggest that some methoxy-3-(2-phenylethoxy)phenyl)-ethylamine monohydro- neuroactive steroids modulate the NMDA response via g chloride (NE-100) and by haloperidol, but not by spiperone. -

Guidelines for Pharmacists Performing Clinical Interventions
Guidelines for pharmacists performing clinical interventions PSA Committed to better health © Pharmaceutical Society of Australia Ltd. 2018 This publication contains material that has been provided by the Pharmaceutical Society of Australia (PSA), and may contain material provided by the Commonwealth and third parties. Copyright in material provided by the Commonwealth or third parties belong to them. PSA owns the copyright in the publication as a whole and all material in the publication that has been developed by PSA. In relation to PSA owned material, no part may be reproduced by any process except in accordance with the provisions of the Copyright Act 1968 (Cwth), or the written permission of PSA. Requests and inquiries regarding permission to use PSA material should be addressed to: Pharmaceutical Society of Australia, PO Box 42, Deakin West ACT 2600. If you would like to use material that has been provided by the Commonwealth or third parties, contact them directly. Disclaimer Neither the PSA, nor any person associated with the preparation of this document, accepts liability for any loss which a user of this document may suffer as a result of reliance on the document and, in particular, for: • use of the Guidelines for a purpose for which they were not intended • any errors or omissions in the Guidelines • any inaccuracy in the information or data on which the Guidelines are based or which is contained in them • any interpretations or opinions stated in, or which may be inferred from, the Guidelines. Notification of any inaccuracy or ambiguity found in this document should be made without delay in order that the issue may be investigated and appropriate action taken. -

(12) United States Patent (10) Patent No.: US 8,778,918 B2 Marchewitz (45) Date of Patent: Jul
US008778918B2 (12) United States Patent (10) Patent No.: US 8,778,918 B2 Marchewitz (45) Date of Patent: Jul. 15, 2014 (54) USE OF 19 NORDHEADERIVATIVES FOR 4,587,235 A 5/1986 Bittler et al. ENHANCING PHYSICAL PERFORMANCE 4,634,694. A 1/1987 Loozen et al. 5,028,631 A 7, 1991 Schwartz et al. 5,079,377 A 1/1992 Yoshihama et al. (71) Applicant: Intellectual Wellness, LLC. Shelby 5,424,463 A 6/1995 Lardy et al. Township, MI (US) 5,521,167 A 5/1996 Gobbini et al. 5,585,371 A 12/1996 Lardy (72) Inventor: Eric Marchewitz, Shelby Township, MI 5,744.462 A 4/1998 Schwartz et al. 5,776,923 A 7, 1998 Labrie (US) 5,804,576 A 9, 1998 Schwartz et al. 5.948,434 A 9, 1999 Labrie (73) Assignee: Intellectual Wellness, LLC. Shelby 6,153,606 A 1 1/2000 Lardy et al. Township, MI (US) 6,368,617 B1 4/2002 Hastings et al. 6,391,868 B2 5, 2002 Arnold (*) Notice: Subject to any disclaimer, the term of this 6,489,313 B1 12/2002 Lardy et al. 6,586.417 B1 7/2003 Abraham patent is extended or adjusted under 35 6,667,299 B1 12/2003 Ahlem et al. U.S.C. 154(b) by 0 days. 6,794.374 B1 9, 2004 Weeks 6,924,274 B2 8/2005 Lardy et al. (21) Appl. No.: 14/050,050 6,964,954 B2 11/2005 Dalko et al. 7,002,028 B2 2/2006 White et al. -

7,8-Dihydroxyflavone and 7,8-Substituted Flavone
(19) TZZ __T (11) EP 2 914 585 B1 (12) EUROPEAN PATENT SPECIFICATION (45) Date of publication and mention (51) Int Cl.: of the grant of the patent: C07D 311/30 (2006.01) C07D 311/28 (2006.01) 07.03.2018 Bulletin 2018/10 A61K 31/353 (2006.01) A61P 25/00 (2006.01) C07D 311/38 (2006.01) C07D 311/36 (2006.01) (21) Application number: 13851584.6 (86) International application number: (22) Date of filing: 01.11.2013 PCT/US2013/067972 (87) International publication number: WO 2014/071134 (08.05.2014 Gazette 2014/19) (54) 7,8-DIHYDROXYFLAVONE AND 7,8-SUBSTITUTED FLAVONE DERIVATIVES, COMPOSITIONS, AND METHODS RELATED THERETO 7,8-DIHYDROXYFLAVON UND 7,8-SUBSTITUIERTE FLAVONDERIVATE, ZUSAMMENSETZUNGEN UND ZUGEHÖRIGE VERFAHREN 7,8-DIHYDROXYFLAVONE ET DÉRIVÉS DE FLAVONE 7,8-SUBSTITUÉE, COMPOSITIONS ET MÉTHODES ASSOCIÉES (84) Designated Contracting States: WO-A2-2011/156479 AL AT BE BG CH CY CZ DE DK EE ES FI FR GB GR HR HU IE IS IT LI LT LU LV MC MK MT NL NO • CHEN, S. ET AL.: MOLECULAR PL PT RO RS SE SI SK SM TR PHARMACOLOGY, vol. 47, 1995, pages 419-424, XP002753952, (30) Priority: 05.11.2012 US 201261722339 P • S.-W. JANG ET AL: "A selective TrkB agonist with 12.07.2013 US 201361845399 P potent neurotrophic activities by 7,8-dihydroxyflavone", PROCEEDINGS OF THE (43) Date of publication of application: NATIONAL ACADEMY OF SCIENCES, vol. 107, 09.09.2015 Bulletin 2015/37 no. 6, 9 February 2010 (2010-02-09), pages 2687-2692, XP055084207, ISSN: 0027-8424, DOI: (73) Proprietor: Emory University 10.1073/pnas.0913572107 Atlanta, GA 30322 (US) • JANG,S-W.