Hyla Cinerea), but May Not Help Small Males
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

R Conradie Orcid.Org 0000-0002-8653-4702
Influence of the invasive fish, Gambusia affinis, on amphibians in the Western Cape R Conradie orcid.org 0000-0002-8653-4702 Dissertation submitted in fulfilment of the requirements for the degree Master of Science in Zoology at the North-West University Supervisor: Prof LH du Preez Co-supervisor: Prof AE Channing Graduation May 2018 23927399 “The whole land is made desolate, but no man lays it to heart.” JEREMIAH 12:11 i DECLARATION I, Roxanne Conradie, declare that this dissertation is my own, unaided work, except where otherwise acknowledged. It is being submitted for the degree of M.Sc. to the North-West University, Potchefstroom. It has not been submitted for any degree or examination at any other university. ____________________ (Roxanne Conradie) ii ACKNOWLEDGEMENTS I would like to express my gratitude to the following persons and organisations, without whose assistance this study would not have been possible: My supervisor Prof. Louis du Preez and co-supervisor Prof. Alan Channing, for guidance, advice, support, and encouragement throughout the duration of this study. Prof Louis, your passion for the biological sciences has been an inspiration to me since undergraduate Zoology classes five years ago. Prof Alan, you were a vital pillar of support for me in the Cape and I am incredibly grateful towards you. Thank you both for all the time and effort you have put into helping me with my work, for all your honest and detailed advice, as well as practical help. It is truly a privilege to have had such outstanding biologists as my mentors. My husband Louis Conradie, for offering up so many weekends in order to help me with fieldwork. -

Integrating Climate Change Vulnerability Assessments Into Adaptation Planning
Integrating climate change vulnerability assessments into adaptation planning A case study using the NatureServe Climate Change Vulnerability Index to inform conservation planning for species in Florida A Report Prepared for the Florida Fish and Wildlife Conservation Commission Natalie Dubois, Astrid Caldas, Judy Boshoven & Aimee Delach Defenders of Wildlife is a national, nonprofit, membership organization dedicated to the protection of all native wild animals and plants in their natural communities. Jamie Rappaport Clark, President Donald Barry, Executive Vice President This report was made possible with the generous support of the Doris Duke Charitable Foundation, the Kresge Foundation and the Educational Foundation of America AUTHORS Natalie Dubois Astrid Caldas Judy Boshoven Aimee Delach With additional input from Amielle DeWan and Kathleen Theoharides PRODUCTION Claire Colegrove © 2011 Defenders of Wildlife, 1130 17th St NW, Washington D.C. 20036 http:/www.defenders.org Disclaimer: This document represents the work and views of the authors and does not necessarily imply endorsement by the Florida Fish and Wildlife Conservation Commission. Suggested citation: Dubois, N., A. Caldas, J. Boshoven, and A. Delach. 2011. Integrating Climate Change Vulnerability Assessments into Adaptation Planning: A Case Study Using the NatureServe Climate Change Vulnerability Index to Inform Conservation Planning for Species in Florida [Final Report]. Defenders of Wildlife, Washington D.C. CONTENTS Executive Summary ................................................................................... -

Newsletter V18-N2
Virginia Herpetological Society Newsletter Volume 18, Number 2 August 2008 President Susan Watson Vice President Secretary/Treasurer David Van Gelder Emily Steele / Pattie Crane Catesbeiana Editor Newsletter Editor Paul Sattler Kory Steele Research Committee VHS Webmaster Joy Ware John White Conservation Committee VHS Merchandise Committee Tim Christensen Pattie Crane Contents VHS Business ………………………...…2 Herp Trivia ……………………………….5 Events …………………………………… 6 Conservation Key……………….............8 Book Review ……………………………. 10 Herpcetera………………………………..11 Online Resources ……………………….12 News …………………………………….. 12 Trivia Answers ………………................ 15 Virginia Literature ………………………. 16 Virginia Native ………………………….. 17 Home Page: http://www.vaherpsociety.com Message Board: http://groups.yahoo.com/group/VaHS Online Store: http://www.cafepress.com/vaherpsociety Virginia Herpetological Society Newsletter 2 VHS Business VHS Business 1) Message from the President 4) Fall Meeting results 2) Elections & Biographies 5) Surveys 3) Website Updates 6) Snake Force One 1) Message from the President Susan Watson – ([email protected]) This has been a very busy 50th year for VHS so far, and there are still so many things to do. I am currently working on finishing up the report for last year’s survey in Charles City and New Kent Counties, in order to get it in the next issue of Catesbeiana. Some of the other officers and I recently had a rather busy day (though not as hectic as last year) at the 2nd annual Reptile Day at the Virginia Museum of Natural History, in Martinsville. In addition, I am getting things lined up for this year’s annual Fall Meeting and 50th Anniversary Celebration. The Meeting and 50th Anniversary is slowly developing into a wonderful event! We will have Dr. -

Data Summary, Monitoring Year 2013
National Park Service U.S. Department of the Interior Natural Resource Stewardship and Science Reptile & Amphibian Monitoring at Jean Lafitte National Historical Park and Preserve, Barataria Preserve Data Summary, Monitoring Year 2013 Natural Resource Data Series NPS/GULN/NRDS—2014/652 ON THE COVER The Copperhead, Agkistrodon contortrix, at Jean Lafitte – Barataria Preserve, 2012 Photograph by: RL Woodman, Gulf Coast Inventory and Monitoring Network Reptile & Amphibian Monitoring at Jean Lafitte National Historical Park and Preserve, Barataria Preserve Data Summary, Monitoring Year 2013 Natural Resource Data Series NPS/GULN/NRDS—2014/652 Robert L. Woodman and William Finney National Park Service Gulf Coast I&M Network 646 Cajundome Blvd Lafayette, LA 70506 April 2014 U.S. Department of the Interior National Park Service Natural Resource Stewardship and Science Fort Collins, Colorado The National Park Service, Natural Resource Stewardship and Science office in Fort Collins, Colorado, publishes a range of reports that address natural resource topics These reports are of interest and applicability to a broad audience in the National Park Service and others in natural resource management, including scientists, conservation and environmental constituencies, and the public. The Natural Resource Data Series is intended for the timely release of basic data sets and data summaries. Care has been taken to assure accuracy of raw data values, but a thorough analysis and interpretation of the data has not been completed. Consequently, the initial analyses of data in this report are provisional and subject to change. All manuscripts in the series receive the appropriate level of peer review to ensure that the information is scientifically credible, technically accurate, appropriately written for the intended audience, and designed and published in a professional manner. -

Occurrence of Lymphedema in Wild-Caught Anurans
bioRxiv preprint doi: https://doi.org/10.1101/336131; this version posted June 9, 2018. The copyright holder for this preprint (which was not certified by peer review) is the author/funder. All rights reserved. No reuse allowed without permission. BIORXIV/2018/336131 Occurrence of Lymphedema in Wild-Caught Anurans Malcolm L. McCallum P.O. Box 150, Langston, OK 73050 email: [email protected] Abstract: Lymphedema is a condition in which the lymph hearts fail to pump fluid from the lymph sacs of anurans and other amphibians. This causes the sacs to fill with fluid and provide the frog with balloon-like swellings or over-all appearance. The condition has previously been connected with various diseases including tadpole edema virus and chytrids. I observed lymphedema in six anuran species (Acris blanchadi*, Anaxyrus fowleri*, Hyla squirrela*, Pseudacris streckeri illinoensis*, Rana sylvatica, Rana sphenocephala* [species with * are species records for lymphedema]). Keywords: lymphedema, Batrachochytrium dendrobatidis, Flavobacterium indologenes, chytrids, inflammation. Introduction Reports of lymphedema-like disorders in anurans are rare in the literature. This disorder, first described in 1915 (Moore, 1915) has been identified as one of the most common abnormalities in captive bred Xenopus. This lymphatic dysfunction most commonly results before or just after metamorphosis. It is characterized by extreme inflammation of tissues giving the frog a ballooned appearance. Apparently the lymph hearts fail to drain the lymph sacs and do not reach maturity (Elkan, 1976). This condition may be an inherited condition due to a recessive gene (Uehlinger, 1965, 1969). Subcutaneous lymph sac structure has been described in some anurans (Carter, 1979). -

Captive Wildlife Regulations, 2021, W-13.12 Reg 5
1 CAPTIVE WILDLIFE, 2021 W-13.12 REG 5 The Captive Wildlife Regulations, 2021 being Chapter W-13.12 Reg 5 (effective June 1, 2021). NOTE: This consolidation is not official. Amendments have been incorporated for convenience of reference and the original statutes and regulations should be consulted for all purposes of interpretation and application of the law. In order to preserve the integrity of the original statutes and regulations, errors that may have appeared are reproduced in this consolidation. 2 W-13.12 REG 5 CAPTIVE WILDLIFE, 2021 Table of Contents PART 1 PART 5 Preliminary Matters Zoo Licences and Travelling Zoo Licences 1 Title 38 Definition for Part 2 Definitions and interpretation 39 CAZA standards 3 Application 40 Requirements – zoo licence or travelling zoo licence PART 2 41 Breeding and release Designations, Prohibitions and Licences PART 6 4 Captive wildlife – designations Wildlife Rehabilitation Licences 5 Prohibition – holding unlisted species in captivity 42 Definitions for Part 6 Prohibition – holding restricted species in captivity 43 Standards for wildlife rehabilitation 7 Captive wildlife licences 44 No property acquired in wildlife held for 8 Licence not required rehabilitation 9 Application for captive wildlife licence 45 Requirements – wildlife rehabilitation licence 10 Renewal 46 Restrictions – wildlife not to be rehabilitated 11 Issuance or renewal of licence on terms and conditions 47 Wildlife rehabilitation practices 12 Licence or renewal term PART 7 Scientific Research Licences 13 Amendment, suspension, -
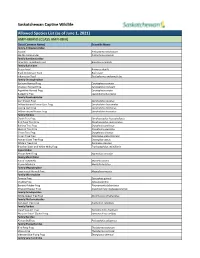
Captive Wildlife Allowed List
Saskatchewan Captive Wildlife Allowed Species List (as of June 1, 2021) AMPHIBIANS (CLASS AMPHIBIA) Class (Common Name) Scientific Name Family Ambystomatidae Axolotl Ambystoma mexicanum Marble Salamander Ambystoma opacum Family Bombinatoridae Oriental Fire-Bellied Toad Bombina orientalis Family Bufonidae Green Toad Anaxyrus debilis Black Indonesian Toad Bufo asper Indonesian Toad Duttaphrynus melanostictus Family Ceratophryidae Surinam Horned Frog Ceratophrys cornuta Chacoan Horned Frog Ceratophrys cranwelli Argentine Horned Frog Ceratophrys ornata Budgett’s Frog Lepidobatrachus laevis Family Dendrobatidae Dart Poison Frog Dendrobates auratus Yellow-banded Poison Dart Frog Dendrobates leucomelas Dyeing Dart Frog Dendrobates tinctorius Yellow-striped Poison Frog Dendrobates truncatus Family Hylidae Clown Tree Frog Dendropsophus leucophyllatus Bird Poop Tree Frog Dendropsophus marmoratus Barking Tree Frog Dryophytes gratiosus Squirrel Tree Frog Dryophytes squirellus Green Tree Frog Dryophytes cinereus Cuban Tree Frog Osteopilus septentrionalis Haitian Giant Tree Frog Osteopilus vastus White’s Tree Frog Ranoidea caerulea Brazilian Black and White Milky Frog Trachycephalus resinifictrix Hyperoliidae African Reed Frog Hyperolius concolor Family Mantellidae Baron’s Mantella Mantella baroni Brown Mantella Mantella betsileo Family Megophryidae Long-nosed Horned Frog Megophrys nasuta Family Microhylidae Tomato Frog Dyscophus guineti Chubby Frog Kaloula pulchra Banded Rubber Frog Phrynomantis bifasciatus Emerald Hopper Frog Scaphiophryne madagascariensis -

Bibliography of the Anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 – 2012
January 2018 Open Access Publishing Volume 13, Monograph 7 A female Western Toad (Anaxyrus boreas) from Garibaldi Provincial Park, British Columbia, Canada. This large bufonid occurs throughout much of Western North America. The IUCN lists it as Near Threatened because it is probably in significant decline (> 30% over 10 years) due to disease.(Photographed by C. Kenneth Dodd). Bibliography of the Anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 – 2012. Monograph 7. C. Kenneth Dodd, Jr. ISSN: 1931-7603 Indexed by: Zoological Record, Scopus, Current Contents / Agriculture, Biology & Environmental Sciences, Journal Citation Reports, Science Citation Index Extended, EMBiology, Biology Browser, Wildlife Review Abstracts, Google Scholar, and is in the Directory of Open Access Journals. BIBLIOGRAPHY OF THE ANURANS OF THE UNITED STATES AND CANADA. VERSION 2, UPDATED AND COVERING THE PERIOD 1709 – 2012. MONOGRAPH 7. C. KENNETH DODD, JR. Department of Wildlife Ecology and Conservation, University of Florida, Gainesville, Florida, USA 32611. Copyright © 2018. C. Kenneth Dodd, Jr. All Rights Reserved. Please cite this monograph as follows: Dodd, C. Kenneth, Jr. 2018. Bibliography of the anurans of the United States and Canada. Version 2, Updated and Covering the Period 1709 - 2012. Herpetological Conservation and Biology 13(Monograph 7):1-328. Table of Contents TABLE OF CONTENTS i PREFACE ii ABSTRACT 1 COMPOSITE BIBLIOGRAPHIC TRIVIA 1 LITERATURE CITED 2 BIBLIOGRAPHY 2 FOOTNOTES 325 IDENTICAL TEXTS 325 CATALOGUE OF NORTH AMERICAN AMPHIBIANS AND REPTILES 326 ADDITIONAL ANURAN-INCLUSIVE BIBLIOGRAPHIES 326 AUTHOR BIOGRAPHY 328 i Preface to Version 2: An Expanded and Detailed Resource. MALCOLM L. -

Appendix 13.A. Wildlife of the St. Johns River Floodplain
Chapter 13. Floodplain Wildlife - Appendices Appendix 13.A. Wildlife of the St. Johns River Floodplain SPECIES DIVERSITY The St. Johns River is a diverse ecosystem with a variety of wildlife. The river’s vertebrate wildlife classes (excluding fish) are the Amphibia (frogs and salamanders), Chelonia (turtles), Crocodylia (alligators), Reptilia (snakes and lizards), Aves (birds), and Mammalia (mammals) (Table 13.A-1). The faunal composition is dominated by birds. Table 13.A–1. Estimated floodplain species composition of the St. Johns River with representation for the non-piscine vertebrate classes. Vertebrate Class Estimated Species Composition Amphibia (frogs, toads, 19 salamanders) Chelonia (turtles) 11 Crocodylia (alligators) 1 Reptilia (snakes and lizards) 23 Aves (birds) 200 Mammalia (mammals) 32 Amphibians The amphibians of the floodplain consist of frogs, toads, and salamanders. These small animals are important components of aquatic and wetland food webs during all their life stages. They are mid-level consumers of invertebrates. Likewise, amphibians are important prey for numerous predators, such as snakes and wading birds. Twelve anuran (frog) species have range distributions that include the St. Johns River floodplain. Additional frog species occur in the larger St. Johns River basin. These species are not included in the wildlife inventory because they lack adaptive mechanisms for surviving the presence of fish, limiting their distributions to isolated wetlands or ephemeral ponds. The vegetated littoral zones of fresh lentic waters are important to the life stages of floodplain frogs. All frogs need standing water for reproduction. Vegetated shallow waters function as sites for oviposition, external fertilization, and nursery areas for free-swimming tadpoles. -

Section 8. Appendices
Section 8. Appendices Appendix 1.1 — Acronyms Terminology AWAP – Arkansas Wildlife Action Plan BMP – Best Management Practice CWCS — Comprehensive Wildlife Conservation Strategy EO — Element Occurrence GIS — Geographic Information Systems SGCN — Species of Greatest Conservation Need LIP — Landowner Incentive Program MOA — Memorandum of Agreement ACWCS — Arkansas Comprehensive Wildlife Conservation Strategy SWG — State Wildlife Grant LTA — Land Type Association WNS — White-nose Syndrome Organizations ADEQ — Arkansas Department of Environmental Quality AGFC — Arkansas Game and Fish Commission AHTD — Arkansas Highway and Transportation Department ANHC — Arkansas Natural Heritage Commission ASU — Arkansas State University ATU — Arkansas Technical University FWS — Fish and Wildlife Service HSU — Henderson State University NRCS — Natural Resources Conservation Service SAU — Southern Arkansas University TNC — The Nature Conservancy UA — University of Arkansas (Fayetteville) UA/Ft. Smith — University of Arkansas at Fort Smith UALR — University of Arkansas at Little Rock UAM — University of Arkansas at Monticello UCA — University of Central Arkansas USFS — United States Forest Service 1581 Appendix 2.1. List of Species of Greatest Conservation Need by Priority Score. List of species of greatest conservation need ranked by Species Priority Score. A higher score implies a greater need for conservation concern and actions. Priority Common Name Scientific Name Taxa Association Score 100 Curtis Pearlymussel Epioblasma florentina curtisii Mussel 100 -

Herp Lab Syllabus 2020 1-3-20
RAT HERPETOLOGY LAB – 1-3-20 LOYOLA UNIVERSITY NEW ORLEANS HERPETOLOGY LAB - BIOL A346 - SPRING 2020 LABORATORY GUIDE Professor: Dr. Robert A. Thomas ([email protected]) Office Hours: TR 2:00-3:15pm; MW 1:00 - 2:15 pm; other times by appointment. If I’m in the office, drop in and inquire if I’m available. THE GOAL: To give you a serious and long-lasting case of herpetitis. LAB PLACE & TIME: The wet lab will be held each Friday in MO 558 from 3:30-6:20 pm. DOOR CODE: 540121 CLASS COMMUNICATION (REQUIRED): I will often communicate with the class via email. Check often (daily) or you will definitely miss important information. Not getting the messages is not a valid excuse – you snooze, you lose. Both of the following must be done by the end of the first week of classes. • CLASS LISTSERV: I have set up a class googlegroup – [email protected]. All announcements and changes as the course progresses may be shared via this googlegroup. Note: You will receive emails from me on this googlegroup only at the address you subscribe from. You may subscribe from more than one email if you wish. Don’t risk losing points by failing to pay attention to this communication system immediately. HOW DO YOU USE THE GOOGLEGROUP? To send an email to the entire class, send it to [email protected]. If you receive an email from this address, clicking <reply all> sends your reply back to the entire class (use caution!). If you hit <reply>, it goes back only to the sender (again, use caution with what you say). -

FOR IMMEDIATE RELEASE National Contact: Carl Walton June 13, 2019 202.268.6539 Mobile 804.402.6702 [email protected]
FOR IMMEDIATE RELEASE National Contact: Carl Walton June 13, 2019 202.268.6539 mobile 804.402.6702 [email protected] Local Contact: Brian Sperry (720) 244-5106 [email protected] usps.com/news Frogs Are Forever U.S. Postal Service Celebrates Four Species with New Forever Stamps What: The U.S. Postal Service issues Frogs, four stamps in a booklet of 20 that feature digital illustrations of four North American frogs: the Pacific tree frog, the northern leopard frog, the American green tree frog and the squirrel tree frog. The First Day of Issue event is free and open to the public. News of the stamp is being shared with the hashtag #FrogStamps. Who: Tom Samra, Vice President, Facilities, U.S. Postal Service When: Tuesday, July 9, at 11 a.m. MT Where: Morrison Knudsen Nature Center 600 S. Walnut Street Boise, ID 83712 RSVP: Dedication ceremony attendees are encouraged to RSVP at usps.com/frogs. Frogs live on every continent except Antarctica; more than 90 species live in the Background: United States, including the four on these stamps. Found throughout the western United States, the tiny Pacific tree frog (Pseudacris regilla) is nicknamed the “Hollywood frog.” Countless television shows and movies have used its rib-bit, rib-bit call in nighttime scenes. The northern leopard frog (Rana pipiens) is commonly identified by its highly distinctive call, a rattle-like snoring noise, followed by several notes described as “chuckling” or “clucking.” Sometimes called the rain frog for its noisy choruses after a warm rain, the American green tree frog (Hyla cinerea) is also dubbed the cowbell frog for the sound of its short call when heard from a distance.