Early Autumn Senescence in Red Maple (Acer Rubrum L.) Is Associated with High Leaf Anthocyanin Content
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Department of Planning and Zoning
Department of Planning and Zoning Subject: Howard County Landscape Manual Updates: Recommended Street Tree List (Appendix B) and Recommended Plant List (Appendix C) - Effective July 1, 2010 To: DLD Review Staff Homebuilders Committee From: Kent Sheubrooks, Acting Chief Division of Land Development Date: July 1, 2010 Purpose: The purpose of this policy memorandum is to update the Recommended Plant Lists presently contained in the Landscape Manual. The plant lists were created for the first edition of the Manual in 1993 before information was available about invasive qualities of certain recommended plants contained in those lists (Norway Maple, Bradford Pear, etc.). Additionally, diseases and pests have made some other plants undesirable (Ash, Austrian Pine, etc.). The Howard County General Plan 2000 and subsequent environmental and community planning publications such as the Route 1 and Route 40 Manuals and the Green Neighborhood Design Guidelines have promoted the desirability of using native plants in landscape plantings. Therefore, this policy seeks to update the Recommended Plant Lists by identifying invasive plant species and disease or pest ridden plants for their removal and prohibition from further planting in Howard County and to add other available native plants which have desirable characteristics for street tree or general landscape use for inclusion on the Recommended Plant Lists. Please note that a comprehensive review of the street tree and landscape tree lists were conducted for the purpose of this update, however, only -

Checklist of Illinois Native Trees
Technical Forestry Bulletin · NRES-102 Checklist of Illinois Native Trees Jay C. Hayek, Extension Forestry Specialist Department of Natural Resources & Environmental Sciences Updated May 2019 This Technical Forestry Bulletin serves as a checklist of Tree species prevalence (Table 2), or commonness, and Illinois native trees, both angiosperms (hardwoods) and gym- county distribution generally follows Iverson et al. (1989) and nosperms (conifers). Nearly every species listed in the fol- Mohlenbrock (2002). Additional sources of data with respect lowing tables† attains tree-sized stature, which is generally to species prevalence and county distribution include Mohlen- defined as having a(i) single stem with a trunk diameter brock and Ladd (1978), INHS (2011), and USDA’s The Plant Da- greater than or equal to 3 inches, measured at 4.5 feet above tabase (2012). ground level, (ii) well-defined crown of foliage, and(iii) total vertical height greater than or equal to 13 feet (Little 1979). Table 2. Species prevalence (Source: Iverson et al. 1989). Based on currently accepted nomenclature and excluding most minor varieties and all nothospecies, or hybrids, there Common — widely distributed with high abundance. are approximately 184± known native trees and tree-sized Occasional — common in localized patches. shrubs found in Illinois (Table 1). Uncommon — localized distribution or sparse. Rare — rarely found and sparse. Nomenclature used throughout this bulletin follows the Integrated Taxonomic Information System —the ITIS data- Basic highlights of this tree checklist include the listing of 29 base utilizes real-time access to the most current and accept- native hawthorns (Crataegus), 21 native oaks (Quercus), 11 ed taxonomy based on scientific consensus. -

Acer Rubrum ‘Red Sunset’ ‘Red Sunset’ Red Maple1 Edward F
Fact Sheet ST-47 November 1993 Acer rubrum ‘Red Sunset’ ‘Red Sunset’ Red Maple1 Edward F. Gilman and Dennis G. Watson2 INTRODUCTION ‘Red Sunset’ and ‘October Glory’ have proven to be the best cultivars of Red Maple for the south (Fig. 1). ‘Red Sunset’ has strong wood and is a vigorous, fast-grower, reaching a height of 50 feet with a spread of 25 to 35 feet. Trees are often seen shorter in the southern part of its range unless located on a wet site. This tree is preferred over Red Maple, Silver Maple or Boxelder when a fast-growing maple is needed, and will take on a pyramidal or oval silhouette. The newly emerging red flowers and fruits signal that spring has come. They appear in December and January in Florida, later in the northern part of its range. Leaves retain an attractive high gloss throughout the growing season. The seeds of ‘Red Sunset’ Red Maple are quite popular with squirrels and birds. GENERAL INFORMATION Scientific name: Acer rubrum ‘Red Sunset’ Pronunciation: AY-ser ROO-brum Common name(s): ‘Red Sunset’ Red Maple Family: Aceraceae Figure 1. Middle-aged ‘Red Sunset’ Red Maple. USDA hardiness zones: 4B through 8 (Fig. 2) Origin: native to North America DESCRIPTION Uses: Bonsai; wide tree lawns (>6 feet wide); medium-sized tree lawns (4-6 feet wide); Height: 45 to 50 feet recommended for buffer strips around parking lots or Spread: 25 to 40 feet for median strip plantings in the highway; near a deck Crown uniformity: symmetrical canopy with a or patio; reclamation plant; screen; shade tree; regular (or smooth) outline, and individuals have more specimen; residential street tree or less identical crown forms Availability: generally available in many areas within Crown shape: oval; upright its hardiness range Crown density: moderate 1. -

Gardenergardener
TheThe AmericanAmerican GARDENERGARDENER TheThe MagazineMagazine ofof thethe AAmericanmerican HorticulturalHorticultural SocietySociety January/February 2005 new plants for 2005 Native Fruits for the Edible Landscape Wildlife-Friendly Gardening Chanticleer: A Jewel of a Garden The Do’s andand Don’tsDon’ts ofof Planting Under Trees contents Volume 84, Number 1 . January / February 2005 FEATURES DEPARTMENTS 5 NOTES FROM RIVER FARM 6 MEMBERS’ FORUM 8 NEWS FROM AHS AHS’s restored White House gates to be centerpiece of Philadelphia Flower Show entrance exhibit, The Growing Connection featured during United Nations World Food Day events, Utah city’s volunteer efforts during America in Bloom competition earned AHS Community Involvement Award, Great Southern Tree Conference is newest AHS partner. 14 AHS PARTNERS IN PROFILE page 22 The Care of Trees brings passion and professionalism to arboriculture. 44 GARDENING BY DESIGN 16 NEW FOR 2005 BY RITA PELCZAR Forget plants—dream of design. A preview of the exciting and intriguing new plant introductions. 46 GARDENER’S NOTEBOOK Gardening trends in 2005, All-America 22 CHANTICLEER BY CAROLE OTTESEN Selections winners, Lenten rose is perennial of the year, wildlife This Philadephia-area garden is being hailed as one of the finest gardening courses small public gardens in America. online, new Cornell Web site allows rating of 26 NATIVE FRUITS BY LEE REICH vegetable varieties, Add beauty and flavor to your landscape with carefree natives like Florida gardens recover from hurricane damage, page 46 beach plum, persimmon, pawpaw, and clove currant. gardeners can help with national bird count. 31 TURNING A GARDEN INTO A COMMUNITY BY JOANNE WOLFE 50 In this first in a series of articles on habitat gardening, learn how to GROWING THE FUTURE create an environment that benefits both gardener and wildlife. -

S M Acer Saccharinum L
S M Acer saccharinum L. bundant in some localities, silver A maple is a common tree, found throughout the state except along the coast. It grows largely on sandy banks along streams, usually attaining a height of 60–80 feet and a diameter of 2–3 feet. The trunk normally separates into 3 or 4 upright secondary stems, devoid of branches for some distance. The branches are long and slender, often pendulous. The bark on young trees is smooth, gray, slightly tinged with red. On old trees, it is reddish-brown, fur- rowed, and separated into large thin scales that are loose at the bottom.Twigs are chestnut brown and shiny. The leaves are opposite, deeply In Maine, silver maple is most five-lobed; and the edges are irregular common along major rivers. and sharply toothed. The upper surface is pale green, the lower, silvery white. They turn a pale yellow in fall. 76 SILVER MAPLE E L P A M The flowers are on very short stalks and in clusters. They are green- ish-yellow or sometimes pinkish, open- ing early, long before the leaves appear. The fruit is paired, winged and ripens in spring. Frequently, one of the pair does not fully develop. The twigs are curved upward at the tip, orange or red-brown above and green below, slender, with a bitter taste and a rank odor when broken. The wood is softer than that Silver maple has large globe-shaped flower of the hard maple, close-grained, not buds and smaller vegetative buds. durable and easily worked. -
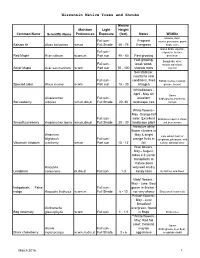
Wisconsin Native Trees and Shrubs
Wisconsin Native Trees and Shrubs Mature Moisture Light Height Common Name Scientific Name Preferences Exposure (feet) Notes Wildlife Grouse, deer, Full sun - Fragrant moose,porcupine, game Balsam fir Abies balsamea wm,m Full Shade 40 - 75 Evergreen birds, mice Game birds, squirrel, Full sun - chipmunk, beaver, Red Maple Acer rubrum w,wm,m Part sun 40 - 60 Fast growing deer,bear Fast growing, Songbirds, deer, Full sun - weak wood, racoon,waterfowl, Silver Maple Acer saccharinum w,wm Part sun 75 - 100 shallow roots squirrel Soil stablizer, neutral to acid Full sun - conditions, fixes Rabbit,moose,muskrat, Specled alder Alnus incana w,wm Part sun 15 - 30 nitrogen grouse, beaver Whiteflowers - April - May An Game Amelanchier Full sun - excellent birds,grouse,skunk,fox, Serviceberry arborea wm,m,dm,d Full Shade 20 -30 landscape tree racoon White flowers - May Orange fall Full sun - color Excellent Birds,bear,squirrel,chipm Smooth juneberry Amelanchier laevis wm,m,dm,d Full Shade 20 - 30 landscape plant unk,deer,moose Attractive white flower clusters in American May & bright Late winter food for Highbush Full sun - orange fruits in songbirds, pheasant, wild Viburnum trilobum cranberry wm,m Part sun 10 - 13' fall turkey, whitetail deer Blue flowers, May - August; takes 2-3 yrs for transplants to mature;does Amorpha very well on dry Leadplant canescens m,dm,d Full sun 1-3 sandy sites Butterflies and Bees Violet flowers - May - June Best Indigobush; False Full sun - grown in thicket - indigo Amorpha fruticosa w,wm,m Full Shade 6 - 12 not very -

Beaver (Castor Canadensis) Impacts on Herbaceous and Woody Vegetation in Southeastern Georgia
Georgia Southern University Digital Commons@Georgia Southern Electronic Theses and Dissertations Graduate Studies, Jack N. Averitt College of Fall 2005 Beaver (Castor Canadensis) Impacts on Herbaceous and Woody Vegetation in Southeastern Georgia Jessica R. Brzyski Follow this and additional works at: https://digitalcommons.georgiasouthern.edu/etd Recommended Citation Brzyski, Jessica R., "Beaver (Castor Canadensis) Impacts on Herbaceous and Woody Vegetation in Southeastern Georgia" (2005). Electronic Theses and Dissertations. 707. https://digitalcommons.georgiasouthern.edu/etd/707 This thesis (open access) is brought to you for free and open access by the Graduate Studies, Jack N. Averitt College of at Digital Commons@Georgia Southern. It has been accepted for inclusion in Electronic Theses and Dissertations by an authorized administrator of Digital Commons@Georgia Southern. For more information, please contact [email protected]. BEAVER (CASTOR CANADENSIS) IMPACTS ON HERBACEOUS AND WOODY VEGETATION IN SOUTHEASTERN GEORGIA by JESSICA R. BRZYSKI (Under the direction of Bruce A. Schulte) ABSTRACT North American beavers are considered ecosystem engineers. Their activities can quickly and drastically alter habitat properties and perhaps permit highly aggressive colonizing plants, notably non-native species, to invade and potentially dominate. This study examined if beavers in southeastern Georgia have an effect on the terrestrial plant community. Sampling areas included beaver modified (N=9) and nearby but relatively non-impacted riparian habitat (N=9) in a matched pairs design. Vegetation surveys were performed in spring and summer. Species richness was calculated for herbs, vines, woody seedlings, and woody vegetation. Richness of herbaceous vegetation was higher at distances closer to shore while richness of large woody vegetation increased with distance from shore. -

Red Maple 15 Gal $72.00 Acer Rubrum
15 Gallon Trees (scroll down for 25 Gal trees) Acer rubrum - 'Autumn Fantasy' Red Maple 15 Gal $72.00 Acer rubrum – 'Red Sunset' Red Maple 15 Gal $72.00 Acer rubrum – 'Sun Valley' Red Maple 15 Gal $72.00 Acer x freemanii - 'Armstrong' Maple 15 Gal $72.00 Acer x freemanii - 'Autumn Blaze' 15 Gal $72.00 Acer x freemanii - 'Sienna' Sienna Glen Maple 15 Gal $72.00 Betula nigra - 'Dura Heat' Riverbirch 15 Gal $72.00 Carpinus betulus - 'Fastigiata' Euro Hornbeam 15 Gal (low branch) $72.00 Cercis canadensis - Eastern Redbud STD 15gl $72.00 Cercis canadensis - Eastern Redbud 15gl $72.00 Chionanthus virginicus - Chinese Fringe tree 15gl $72.00 Cornus florida - White flowering dogwood 15gl $72.00 X cupressocyparis leylandii - 'Leyland' 15 Gal $72.00 Gleditsia triacanthos - 'Shademaster' Honey Locust 15gl $72.00 Hydrangea tardiva 15gl $72.00 Ilex x Nellie R Stevens Holly 15 Gal $72.00 Lagerstroemia -Muskogee 15 Gal $72.00 Lagerstroemia - Natchez 15 Gal $72.00 Malus 'Prariefire' 15gl $72.00 Malus 'Profusion' 15gl $72.00 Malus 'Starlite' 15gl $72.00 Magnolia Virginiana - Sweetbay magnolia 15gl $72.00 Ostrya virginiana - Hophornbeam 15 Gal $72.00 Platanus occidentalis - American Sycamore 15 Gal $72.00 Platanus x acerfolia - 'Bloodgood' London Planetree 15 Gal $72.00 Platanus x acerfolia - 'Exclaimation' London Planetree 15 Gal $72.00 Prunus cerasifera - 'Krauter Vesuvius'' Plum 15gl $72.00 Prunus cerasifera - 'Newport' Plum 15gl $72.00 Prunus virginiana - 'Canada Red' Cherry 15 Gal $72.00 Prunus x incamp - 'Okame' Cherry 15 Gal $72.00 Prunus x snofozam -

Acer Saccharinum: Silver Maple1 Edward F
ENH-207 Acer saccharinum: Silver Maple1 Edward F. Gilman, Dennis G. Watson, Ryan W. Klein, Andrew K. Koeser, Deborah R. Hilbert and Drew C. McLean2 Introduction Silver maple has a vase shape and is a rapidly growing, fairly weak-wooded tree that reaches a height of 60 to 80 feet with a 5- to 6- foot diameter trunk on a moist site. The tree is useful in wet areas, transplants easily, and can grow where few others can. It should be saved for planting in wet areas or where nothing else will thrive. Roots often grow on the surface of the soil, making mowing grass difficult under the canopy. They also are aggressive, growing into septic tank drain fields and into broken water and sewer pipes. It is also hard to plant shrubs and other plants beneath the branches due to the dense root system. General Information Scientific name: Acer saccharinum Pronunciation: AY-ser sack-uh-RYE-num Common name(s): silver maple Family: Sapindaceae USDA hardiness zones: 3A through 9B (Figure 2) Origin: native to eastern half of the United States and southeastern Canada UF/IFAS Invasive Assessment Status: native Uses: urban tolerant; shade; reclamation Figure 1. Full Form - Acer saccharinum: silver maple Credits: Gitta Hasing, UF/IFAS 1. This document is ENH-207, one of a series of the Environmental Horticulture Department, UF/IFAS Extension. Original publication date November 1993. Revised December 2018. Visit the EDIS website at https://edis.ifas.ufl.edu for the currently supported version of this publication. 2. Edward F. Gilman, professor emeritus, Environmental Horticulture Department; Dennis G. -

October 1961 , Volume 40, Number 4 305
TIIE .A:M:ERICA.N ~GAZINE AMERICAN HORTICULTURAL SOCIETY A union of the Amej'ican HOTticu~tural Society and the AmeTican HOTticultural Council 1600 BLADENSBURG ROAD, NORTHEAST. WASHINGTON 2, D. C. For United Horticulture *** to accumulate, increase, and disseminate horticultural intOTmation B. Y. MORRISON, Editor Directors Terms Expiring 1961 JAMES R. HARLOW, Managing Editor STUART M. ARMSTRONG Maryland Editorial Committee JOH N L. CREECH . Maryland W. H . HODGE, Chairman WILLIAM H. FREDERICK, JR. Delaware JOH N L. CREECH FRANCIS PATTESON-KNIGHT FREDERIC P. LEE Virginia DONALD WYMAN CONRAD B. LINK Massachusetts CURTIS MAY T erms Expiring 1962 FREDERICK G . MEYER FREDERIC P. LEE WILBUR H . YOUNGMAN Maryland HENRY T . SKINNER District of Columbia OfJiceTS GEORGE H. SPALDING California PRESIDENT RICHARD P. WHITE DONAlJD WYMAN Distj'ict of Columbia Jamaica Plain, Massachusetts ANNE WERTSNER WOOD Pennsylvania FIRST VICE· PRESIDENT Ternu Expiring 1963 ALBERT J . IRVING New l'm'k, New York GRETCHEN HARSHBARGER Iowa SECOND VICE-PRESIDENT MARY W. M. HAKES Maryland ANNE WERTSNER W ' OOD FREDERIC HEUTTE Swarthmore, Pennsylvania Virginia W . H. HODGE SECRETARY-TREASURER OLIVE E. WEATHERELL ALBERT J . IRVING Washington, D, C. New York The Ame"ican Horticultural Magazine is the official publication of the American Horticultural Society and is issued four times a year during the quarters commencing with January, April, July and October. It is devoted to the dissemination of knowledge in the science and art of growing ornamental plants, fruits, vegetables, and related subjects. Original papers increasing the historical, varietal, and cultural knowledges of plant materials of economic and aesthetic importance are welcomed and will be published as early as possible. -

Red Maple (Acer Rubrum) Plant Family: Aceraceae
BWSR Featured Plant Name: Red Maple (Acer rubrum) Plant Family: Aceraceae Statewide Wetland Acer rubrum is hard to miss in the fall landscape Indicator Status: with its bright red fall color. Blooming from FAC April to May and growing up to 90 feet tall, this native tree provides habitat in moist forests for cavity nesters like wood ducks, woodpeckers, and chickadees and is an important early season source of pollen and nectar for pollinators. The species also plays an important role in stabilizing soils along rivers and streams. Red maple, fall color Red female flowers above and Identification newly developing seeds below Gray bark, rounded buds and simple, opposite leaves aid in the identification of red maple. It tends to have a relativley open growth form with main branches that are closer to the ground than many other tree species. Its leaves are around four inches long, palmate, with three shallow lobes and five main veins radiating from the base. Its red or yellowish flowers bloom early in the spring before the leaves develop. When it matures in early summer, the red, yellow or reddish-brown samaras are distributed by wind and root easily by a large taproot, followed by smaller lateral roots. Range In the Eastern Deciduous Forest, red maple is one of the most successful species due to aggressive seeding and an ability to tolerant a wide variety of site conditions. Red maple prefers wet sites such as floodplains, river valleys, swamps, forested bogs, and wooded bluffs. However, the species is widespread throughout Minnesota and North America, from Newfoundland and Nova Scotia south to Texas and east to Florida. -

Landscaping Near Black Walnut Trees
Selecting juglone-tolerant plants Landscaping Near Black Walnut Trees Black walnut trees (Juglans nigra) can be very attractive in the home landscape when grown as shade trees, reaching a potential height of 100 feet. The walnuts they produce are a food source for squirrels, other wildlife and people as well. However, whether a black walnut tree already exists on your property or you are considering planting one, be aware that black walnuts produce juglone. This is a natural but toxic chemical they produce to reduce competition for resources from other plants. This natural self-defense mechanism can be harmful to nearby plants causing “walnut wilt.” Having a walnut tree in your landscape, however, certainly does not mean the landscape will be barren. Not all plants are sensitive to juglone. Many trees, vines, shrubs, ground covers, annuals and perennials will grow and even thrive in close proximity to a walnut tree. Production and Effect of Juglone Toxicity Juglone, which occurs in all parts of the black walnut tree, can affect other plants by several means: Stems Through root contact Leaves Through leakage or decay in the soil Through falling and decaying leaves When rain leaches and drips juglone from leaves Nuts and hulls and branches onto plants below. Juglone is most concentrated in the buds, nut hulls and All parts of the black walnut tree produce roots and, to a lesser degree, in leaves and stems. Plants toxic juglone to varying degrees. located beneath the canopy of walnut trees are most at risk. In general, the toxic zone around a mature walnut tree is within 50 to 60 feet of the trunk, but can extend to 80 feet.