Characterization of the Endothelial Cell Cytoskeleton Following HLA Class I Ligation
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Gene Symbol Gene Description ACVR1B Activin a Receptor, Type IB
Table S1. Kinase clones included in human kinase cDNA library for yeast two-hybrid screening Gene Symbol Gene Description ACVR1B activin A receptor, type IB ADCK2 aarF domain containing kinase 2 ADCK4 aarF domain containing kinase 4 AGK multiple substrate lipid kinase;MULK AK1 adenylate kinase 1 AK3 adenylate kinase 3 like 1 AK3L1 adenylate kinase 3 ALDH18A1 aldehyde dehydrogenase 18 family, member A1;ALDH18A1 ALK anaplastic lymphoma kinase (Ki-1) ALPK1 alpha-kinase 1 ALPK2 alpha-kinase 2 AMHR2 anti-Mullerian hormone receptor, type II ARAF v-raf murine sarcoma 3611 viral oncogene homolog 1 ARSG arylsulfatase G;ARSG AURKB aurora kinase B AURKC aurora kinase C BCKDK branched chain alpha-ketoacid dehydrogenase kinase BMPR1A bone morphogenetic protein receptor, type IA BMPR2 bone morphogenetic protein receptor, type II (serine/threonine kinase) BRAF v-raf murine sarcoma viral oncogene homolog B1 BRD3 bromodomain containing 3 BRD4 bromodomain containing 4 BTK Bruton agammaglobulinemia tyrosine kinase BUB1 BUB1 budding uninhibited by benzimidazoles 1 homolog (yeast) BUB1B BUB1 budding uninhibited by benzimidazoles 1 homolog beta (yeast) C9orf98 chromosome 9 open reading frame 98;C9orf98 CABC1 chaperone, ABC1 activity of bc1 complex like (S. pombe) CALM1 calmodulin 1 (phosphorylase kinase, delta) CALM2 calmodulin 2 (phosphorylase kinase, delta) CALM3 calmodulin 3 (phosphorylase kinase, delta) CAMK1 calcium/calmodulin-dependent protein kinase I CAMK2A calcium/calmodulin-dependent protein kinase (CaM kinase) II alpha CAMK2B calcium/calmodulin-dependent -

Supplementary Figure S1. Intracellular Ca2+ Levels Following Decursin Treatment in F11 Cells in the Presence of Menthol
Supplementary Figure S1. Intracellular Ca2+ levels following decursin treatment in F11 cells in the presence of menthol (A) Intracellular Ca2+ levels after treatment with decursin every 3 s. The red arrow indicates the duration of treatment with 200 μM of menthol and decursin. NC: The negative control treated with DMSO only; PC: The positive control treated with 200 μM menthol without decursin. (B) Average intracellular Ca2+ levels after treatment with decursin. The average was quantified from the normalized Δ340/380 ratio for 10 cycles after treatment with the decursin solution at the 10th cycle, as shown in Fig. 1A. The normalized Δ340/380 ratio was calculated using the following for- mula: [ratio of fluorescence intensity at 510 nm (emission) to that at 340 nm (excitation)]/[ratio of fluorescence intensity at 510 nm (emission) to that at a wavelength of 380 nm (excitation)]. Cells 2021, 10, 547. https://doi.org/10.3390/cells10030547 www.mdpi.com/journal/cells Cells 2021, 10, 547 2 of 5 Table S1. List of protein targets of decursin detected by the SwissTargetPrediction web tool Common Target Uniprot ID ChEMBL ID Target Class Probability name Poly [ADP-ribose] polymerase-1 PARP1 P09874 CHEMBL3105 Enzyme 0.104671941 N-acylsphingosine-amidohydro- NAAA Q02083 CHEMBL4349 Enzyme 0.104671941 lase Acid ceramidase ASAH1 Q13510 CHEMBL5463 Enzyme 0.104671941 Family A G protein- Neuropeptide Y receptor type 5 NPY5R Q15761 CHEMBL4561 0.104671941 coupled receptor Family A G protein- Melatonin receptor 1A MTNR1A P48039 CHEMBL1945 0.104671941 coupled -
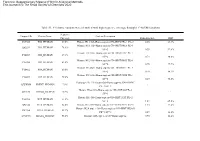
Table S1. 49 Histone Variants Were Identified with High Sequence Coverage Through LC-MS/MS Analysis Electronic Supplementary
Electronic Supplementary Material (ESI) for Analytical Methods. This journal is © The Royal Society of Chemistry 2020 Table S1. 49 histone variants were identified with high sequence coverage through LC-MS/MS analysis Sequence Uniprot IDs Protein Name Protein Description Coverage Ratio E2+/E2- RSD P07305 H10_HUMAN 67.5% Histone H1.0 OS=Homo sapiens GN=H1F0 PE=1 SV=3 4.85 23.3% Histone H1.1 OS=Homo sapiens GN=HIST1H1A PE=1 Q02539 H11_HUMAN 74.4% SV=3 0.35 92.6% Histone H1.2 OS=Homo sapiens GN=HIST1H1C PE=1 P16403 H12_HUMAN 67.1% SV=2 0.73 80.6% Histone H1.3 OS=Homo sapiens GN=HIST1H1D PE=1 P16402 H13_HUMAN 63.8% SV=2 0.75 77.7% Histone H1.4 OS=Homo sapiens GN=HIST1H1E PE=1 P10412 H14_HUMAN 69.0% SV=2 0.70 80.3% Histone H1.5 OS=Homo sapiens GN=HIST1H1B PE=1 P16401 H15_HUMAN 79.6% SV=3 0.29 98.3% Testis-specific H1 histone OS=Homo sapiens GN=H1FNT Q75WM6 H1FNT_HUMAN 7.8% \ \ PE=2 SV=3 Histone H1oo OS=Homo sapiens GN=H1FOO PE=2 Q8IZA3 H1FOO_HUMAN 5.2% \ \ SV=1 Histone H1t OS=Homo sapiens GN=HIST1H1T PE=2 P22492 H1T_HUMAN 31.4% SV=4 1.42 65.0% Q92522 H1X_HUMAN 82.6% Histone H1x OS=Homo sapiens GN=H1FX PE=1 SV=1 1.15 33.2% Histone H2A type 1 OS=Homo sapiens GN=HIST1H2AG P0C0S8 H2A1_HUMAN 99.2% PE=1 SV=2 0.57 26.8% Q96QV6 H2A1A_HUMAN 58.0% Histone H2A type 1-A OS=Homo sapiens 0.90 11.2% GN=HIST1H2AA PE=1 SV=3 Histone H2A type 1-B/E OS=Homo sapiens P04908 H2A1B_HUMAN 99.2% GN=HIST1H2AB PE=1 SV=2 0.92 30.2% Histone H2A type 1-C OS=Homo sapiens Q93077 H2A1C_HUMAN 100.0% GN=HIST1H2AC PE=1 SV=3 0.76 27.6% Histone H2A type 1-D OS=Homo sapiens P20671 -

A Cell Line P53 Mutation Type UM
A Cell line p53 mutation Type UM-SCC 1 wt UM-SCC5 Exon 5, 157 GTC --> TTC Missense mutation by transversion (Valine --> Phenylalanine UM-SCC6 wt UM-SCC9 wt UM-SCC11A wt UM-SCC11B Exon 7, 242 TGC --> TCC Missense mutation by transversion (Cysteine --> Serine) UM-SCC22A Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC22B Exon 6, 220 TAT --> TGT Missense mutation by transition (Tyrosine --> Cysteine) UM-SCC38 Exon 5, 132 AAG --> AAT Missense mutation by transversion (Lysine --> Asparagine) UM-SCC46 Exon 8, 278 CCT --> CGT Missense mutation by transversion (Proline --> Alanine) B 1 Supplementary Methods Cell Lines and Cell Culture A panel of ten established HNSCC cell lines from the University of Michigan series (UM-SCC) was obtained from Dr. T. E. Carey at the University of Michigan, Ann Arbor, MI. The UM-SCC cell lines were derived from eight patients with SCC of the upper aerodigestive tract (supplemental Table 1). Patient age at tumor diagnosis ranged from 37 to 72 years. The cell lines selected were obtained from patients with stage I-IV tumors, distributed among oral, pharyngeal and laryngeal sites. All the patients had aggressive disease, with early recurrence and death within two years of therapy. Cell lines established from single isolates of a patient specimen are designated by a numeric designation, and where isolates from two time points or anatomical sites were obtained, the designation includes an alphabetical suffix (i.e., "A" or "B"). The cell lines were maintained in Eagle's minimal essential media supplemented with 10% fetal bovine serum and penicillin/streptomycin. -

AGC Kinases in Mtor Signaling, in Mike Hall and Fuyuhiko Tamanoi: the Enzymes, Vol
Provided for non-commercial research and educational use only. Not for reproduction, distribution or commercial use. This chapter was originally published in the book, The Enzymes, Vol .27, published by Elsevier, and the attached copy is provided by Elsevier for the author's benefit and for the benefit of the author's institution, for non-commercial research and educational use including without limitation use in instruction at your institution, sending it to specific colleagues who know you, and providing a copy to your institution’s administrator. All other uses, reproduction and distribution, including without limitation commercial reprints, selling or licensing copies or access, or posting on open internet sites, your personal or institution’s website or repository, are prohibited. For exceptions, permission may be sought for such use through Elsevier's permissions site at: http://www.elsevier.com/locate/permissionusematerial From: ESTELA JACINTO, AGC Kinases in mTOR Signaling, In Mike Hall and Fuyuhiko Tamanoi: The Enzymes, Vol. 27, Burlington: Academic Press, 2010, pp.101-128. ISBN: 978-0-12-381539-2, © Copyright 2010 Elsevier Inc, Academic Press. Author's personal copy 7 AGC Kinases in mTOR Signaling ESTELA JACINTO Department of Physiology and Biophysics UMDNJ-Robert Wood Johnson Medical School, Piscataway New Jersey, USA I. Abstract The mammalian target of rapamycin (mTOR), a protein kinase with homology to lipid kinases, orchestrates cellular responses to growth and stress signals. Various extracellular and intracellular inputs to mTOR are known. mTOR processes these inputs as part of two mTOR protein com- plexes, mTORC1 or mTORC2. Surprisingly, despite the many cellular functions that are linked to mTOR, there are very few direct mTOR substrates identified to date. -

Herpes Simplex Virus Blocks Host Transcription Termination Via the Bimodal Activities of ICP27
ARTICLE https://doi.org/10.1038/s41467-019-14109-x OPEN Herpes simplex virus blocks host transcription termination via the bimodal activities of ICP27 Xiuye Wang 1, Thomas Hennig2, Adam W. Whisnant 2, Florian Erhard 2, Bhupesh K. Prusty 2, Caroline C. Friedel 3, Elmira Forouzmand4,5, William Hu1, Luke Erber 6, Yue Chen6, Rozanne M. Sandri-Goldin 1*, Lars Dölken 2,7* & Yongsheng Shi1* Infection by viruses, including herpes simplex virus-1 (HSV-1), and cellular stresses cause 1234567890():,; widespread disruption of transcription termination (DoTT) of RNA polymerase II (RNAPII) in host genes. However, the underlying mechanisms remain unclear. Here, we demonstrate that the HSV-1 immediate early protein ICP27 induces DoTT by directly binding to the essential mRNA 3’ processing factor CPSF. It thereby induces the assembly of a dead-end 3’ processing complex, blocking mRNA 3’ cleavage. Remarkably, ICP27 also acts as a sequence- dependent activator of mRNA 3’ processing for viral and a subset of host transcripts. Our results unravel a bimodal activity of ICP27 that plays a key role in HSV-1-induced host shutoff and identify CPSF as an important factor that mediates regulation of transcription termination. These findings have broad implications for understanding the regulation of transcription termination by other viruses, cellular stress and cancer. 1 Department of Microbiology and Molecular Genetics, School of Medicine, University of California, Irvine, Irvine, CA 92697, USA. 2 Institute for Virology and Immunobiology, Julius-Maximilians-University Würzburg, Würzburg, Germany. 3 Institute of Informatics, Ludwig-Maximilians-Universität München, München, Germany. 4 Institute for Genomics and Bioinformatics, University of California, Irvine, Irvine, CA 92697, USA. -

Protein Kinase D1 Regulates Hypoxic Metabolism Through HIF-1Α and Glycolytic Enzymes Incancer Cells
ONCOLOGY REPORTS 40: 1073-1082, 2018 Protein kinase D1 regulates hypoxic metabolism through HIF-1α and glycolytic enzymes incancer cells JIAO CHEN*, BOMIAO CUI*, YAPING FAN*, XIAOYING LI, QIAN LI, YUE DU, YUN FENG and PING ZHANG State Key Laboratory of Oral Diseases, West China Hospital of Stomatology, Sichuan University, Chengdu, Sichuan 610041, P.R. China Received October 7, 2017; Accepted April 3, 2018 DOI: 10.3892/or.2018.6479 Abstract. Protein kinase D1 (PKD1), one of the protein Introduction kinase D (PKD) family members, plays a prominent role in multiple bio-behaviors of cancer cells. Low pH and hypoxia Oral cancer is a major public health issue and a social chal- are unique characteristics of the tumor microenvironment. lenge. Tongue squamous cell carcinoma (TSCC) is the most The aim of this study was to investigate the role and mecha- aggressive type of oral cancer. Environmental factors, genetics nism of PKD1 in regulating metabolism in the human tongue and immune status have been confirmed to be related to the squamous cell carcinoma (TSCC) cell line SCC25 under a tumorigenesis of tongue cancer (1). The tumor microenviron- hypoxic condition, as well as growth and apoptosis. Here, we ment, which is composed of complex components including found that hypoxia not only induced the expression of HIF-1α, stem cells, fibroblasts, immune cells and their secreted factors, but also induced the expression and activation of PKD1. is critical to the initiation, development and maintenance of Moreover, we inhibited the expression of PKD1 by shRNA tumorigenesis (1). As the ‘residence niche’ of cancer cells, the interference, and the growth of SCC25 cells under hypoxia was composition and structure of the tumor microenvironment significantly decreased, as well as the expression of HIF-1α, not only affects the bio-behavior, but also determines the while the percentage of apoptotic SCC25 cells was increased. -

Phosphatidylinositol-3-Kinase Related Kinases (Pikks) in Radiation-Induced Dna Damage
Mil. Med. Sci. Lett. (Voj. Zdrav. Listy) 2012, vol. 81(4), p. 177-187 ISSN 0372-7025 DOI: 10.31482/mmsl.2012.025 REVIEW ARTICLE PHOSPHATIDYLINOSITOL-3-KINASE RELATED KINASES (PIKKS) IN RADIATION-INDUCED DNA DAMAGE Ales Tichy 1, Kamila Durisova 1, Eva Novotna 1, Lenka Zarybnicka 1, Jirina Vavrova 1, Jaroslav Pejchal 2, Zuzana Sinkorova 1 1 Department of Radiobiology, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic 2 Centrum of Advanced Studies, Faculty of Health Sciences in Hradec Králové, University of Defence in Brno, Czech Republic. Received 5 th September 2012. Revised 27 th November 2012. Published 7 th December 2012. Summary This review describes a drug target for cancer therapy, family of phosphatidylinositol-3 kinase related kinases (PIKKs), and it gives a comprehensive review of recent information. Besides general information about phosphatidylinositol-3 kinase superfamily, it characterizes a DNA-damage response pathway since it is monitored by PIKKs. Key words: PIKKs; ATM; ATR; DNA-PK; Ionising radiation; DNA-repair ABBREVIATIONS therapy and radiation play a pivotal role. Since cancer is one of the leading causes of death worldwide, it is DSB - double stand breaks, reasonable to invest time and resources in the enligh - IR - ionising radiation, tening of mechanisms, which underlie radio-resis - p53 - TP53 tumour suppressors, tance. PI - phosphatidylinositol. The aim of this review is to describe the family INTRODUCTION of phosphatidyinositol 3-kinases (PI3K) and its func - tional subgroup - phosphatidylinositol-3-kinase rela - An efficient cancer treatment means to restore ted kinases (PIKKs) and their relation to repairing of controlled tissue growth via interfering with cell sig - radiation-induced DNA damage. -

A Novel Kinase Inhibitor Establishes a Predominant Role for Protein Kinase D As a Cardiac Class Iia Histone Deacetylase Kinase
View metadata, citation and similar papers at core.ac.uk brought to you by CORE provided by Elsevier - Publisher Connector FEBS Letters 584 (2010) 631–637 journal homepage: www.FEBSLetters.org A novel kinase inhibitor establishes a predominant role for protein kinase D as a cardiac class IIa histone deacetylase kinase Lauren Monovich a,*, Richard B. Vega a, Erik Meredith a, Karl Miranda a, Chang Rao a, Michael Capparelli a, Douglas D. Lemon b, Dillon Phan b, Keith A. Koch b, Joseph A. Chapo b, David B. Hood b, Timothy A. McKinsey b,* a Novartis Institutes for Biomedical Research, 3333 Walnut Street, Boulder, CO 80301, United States b Gilead Colorado, Inc., 3333 Walnut Street, Boulder, CO 80301, United States article info abstract Article history: Class IIa histone deacetylases (HDACs) repress genes involved in pathological cardiac hypertrophy. Received 13 November 2009 The anti-hypertrophic action of class IIa HDACs is overcome by signals that promote their phosphor- Revised 8 December 2009 ylation-dependent nuclear export. Several kinases have been shown to phosphorylate class IIa Accepted 11 December 2009 HDACs, including calcium/calmodulin-dependent protein kinase (CaMK), protein kinase D (PKD) Available online 14 December 2009 and G protein-coupled receptor kinase (GRK). However, the identity of the kinase(s) responsible Edited by Ivan Sadowski for phosphorylating class IIa HDACs during cardiac hypertrophy has remained controversial. We describe a novel and selective small molecule inhibitor of PKD, bipyridyl PKD inhibitor (BPKDi). BPKDi blocks signal-dependent phosphorylation and nuclear export of class IIa HDACs in cardio- Keywords: Kinase myocytes and concomitantly suppresses hypertrophy of these cells. -

Profiling Data
Compound Name DiscoveRx Gene Symbol Entrez Gene Percent Compound Symbol Control Concentration (nM) JNK-IN-8 AAK1 AAK1 69 1000 JNK-IN-8 ABL1(E255K)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317I)-nonphosphorylated ABL1 87 1000 JNK-IN-8 ABL1(F317I)-phosphorylated ABL1 100 1000 JNK-IN-8 ABL1(F317L)-nonphosphorylated ABL1 65 1000 JNK-IN-8 ABL1(F317L)-phosphorylated ABL1 61 1000 JNK-IN-8 ABL1(H396P)-nonphosphorylated ABL1 42 1000 JNK-IN-8 ABL1(H396P)-phosphorylated ABL1 60 1000 JNK-IN-8 ABL1(M351T)-phosphorylated ABL1 81 1000 JNK-IN-8 ABL1(Q252H)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(Q252H)-phosphorylated ABL1 56 1000 JNK-IN-8 ABL1(T315I)-nonphosphorylated ABL1 100 1000 JNK-IN-8 ABL1(T315I)-phosphorylated ABL1 92 1000 JNK-IN-8 ABL1(Y253F)-phosphorylated ABL1 71 1000 JNK-IN-8 ABL1-nonphosphorylated ABL1 97 1000 JNK-IN-8 ABL1-phosphorylated ABL1 100 1000 JNK-IN-8 ABL2 ABL2 97 1000 JNK-IN-8 ACVR1 ACVR1 100 1000 JNK-IN-8 ACVR1B ACVR1B 88 1000 JNK-IN-8 ACVR2A ACVR2A 100 1000 JNK-IN-8 ACVR2B ACVR2B 100 1000 JNK-IN-8 ACVRL1 ACVRL1 96 1000 JNK-IN-8 ADCK3 CABC1 100 1000 JNK-IN-8 ADCK4 ADCK4 93 1000 JNK-IN-8 AKT1 AKT1 100 1000 JNK-IN-8 AKT2 AKT2 100 1000 JNK-IN-8 AKT3 AKT3 100 1000 JNK-IN-8 ALK ALK 85 1000 JNK-IN-8 AMPK-alpha1 PRKAA1 100 1000 JNK-IN-8 AMPK-alpha2 PRKAA2 84 1000 JNK-IN-8 ANKK1 ANKK1 75 1000 JNK-IN-8 ARK5 NUAK1 100 1000 JNK-IN-8 ASK1 MAP3K5 100 1000 JNK-IN-8 ASK2 MAP3K6 93 1000 JNK-IN-8 AURKA AURKA 100 1000 JNK-IN-8 AURKA AURKA 84 1000 JNK-IN-8 AURKB AURKB 83 1000 JNK-IN-8 AURKB AURKB 96 1000 JNK-IN-8 AURKC AURKC 95 1000 JNK-IN-8 -

PIM2-Mediated Phosphorylation of Hexokinase 2 Is Critical for Tumor Growth and Paclitaxel Resistance in Breast Cancer
Oncogene (2018) 37:5997–6009 https://doi.org/10.1038/s41388-018-0386-x ARTICLE PIM2-mediated phosphorylation of hexokinase 2 is critical for tumor growth and paclitaxel resistance in breast cancer 1 1 1 1 1 2 2 3 Tingting Yang ● Chune Ren ● Pengyun Qiao ● Xue Han ● Li Wang ● Shijun Lv ● Yonghong Sun ● Zhijun Liu ● 3 1 Yu Du ● Zhenhai Yu Received: 3 December 2017 / Revised: 30 May 2018 / Accepted: 31 May 2018 / Published online: 9 July 2018 © The Author(s) 2018. This article is published with open access Abstract Hexokinase-II (HK2) is a key enzyme involved in glycolysis, which is required for breast cancer progression. However, the underlying post-translational mechanisms of HK2 activity are poorly understood. Here, we showed that Proviral Insertion in Murine Lymphomas 2 (PIM2) directly bound to HK2 and phosphorylated HK2 on Thr473. Biochemical analyses demonstrated that phosphorylated HK2 Thr473 promoted its protein stability through the chaperone-mediated autophagy (CMA) pathway, and the levels of PIM2 and pThr473-HK2 proteins were positively correlated with each other in human breast cancer. Furthermore, phosphorylation of HK2 on Thr473 increased HK2 enzyme activity and glycolysis, and 1234567890();,: 1234567890();,: enhanced glucose starvation-induced autophagy. As a result, phosphorylated HK2 Thr473 promoted breast cancer cell growth in vitro and in vivo. Interestingly, PIM2 kinase inhibitor SMI-4a could abrogate the effects of phosphorylated HK2 Thr473 on paclitaxel resistance in vitro and in vivo. Taken together, our findings indicated that PIM2 was a novel regulator of HK2, and suggested a new strategy to treat breast cancer. Introduction ATP molecules. -

Genome-Wide Screen of Cell-Cycle Regulators in Normal and Tumor Cells
bioRxiv preprint doi: https://doi.org/10.1101/060350; this version posted June 23, 2016. The copyright holder for this preprint (which was not certified by peer review) is the author/funder, who has granted bioRxiv a license to display the preprint in perpetuity. It is made available under aCC-BY-NC-ND 4.0 International license. Genome-wide screen of cell-cycle regulators in normal and tumor cells identifies a differential response to nucleosome depletion Maria Sokolova1, Mikko Turunen1, Oliver Mortusewicz3, Teemu Kivioja1, Patrick Herr3, Anna Vähärautio1, Mikael Björklund1, Minna Taipale2, Thomas Helleday3 and Jussi Taipale1,2,* 1Genome-Scale Biology Program, P.O. Box 63, FI-00014 University of Helsinki, Finland. 2Science for Life laboratory, Department of Biosciences and Nutrition, Karolinska Institutet, SE- 141 83 Stockholm, Sweden. 3Science for Life laboratory, Division of Translational Medicine and Chemical Biology, Department of Medical Biochemistry and Biophysics, Karolinska Institutet, S-171 21 Stockholm, Sweden To identify cell cycle regulators that enable cancer cells to replicate DNA and divide in an unrestricted manner, we performed a parallel genome-wide RNAi screen in normal and cancer cell lines. In addition to many shared regulators, we found that tumor and normal cells are differentially sensitive to loss of the histone genes transcriptional regulator CASP8AP2. In cancer cells, loss of CASP8AP2 leads to a failure to synthesize sufficient amount of histones in the S-phase of the cell cycle, resulting in slowing of individual replication forks. Despite this, DNA replication fails to arrest, and tumor cells progress in an elongated S-phase that lasts several days, finally resulting in death of most of the affected cells.