Pyropheophytin a in Soft Deodorized Olive Oils
Total Page:16
File Type:pdf, Size:1020Kb

Load more
Recommended publications
-

Universidad De Jaén Gasification Applied to The
UNIVERSIDAD DE JAÉN ESCUELA POLITÉCNICA SUPERIOR DEPARTAMENTO DE INGENIERÍA ELÉCTRICA TESIS DOCTORAL GASIFICATION APPLIED TO THE VALORIZATION OF OLIVE GROVE AND OLIVE MILL RESIDUES PRESENTADA POR: BÁRBARA DE MENA PARDO DIRIGIDA POR: DR. D. DAVID VERA CANDEAS DR. D. FRANCISCO JURADO MELGUIZO JAÉN, 7 DE ABRIL DE 2017 ISBN 978-84-9159-076-7 Gasification applied to the valorization of olive grove and olive mill residues 2 Gasification applied to the valorization of olive grove and olive mill residues Chapter 1. Introduction. Objectives and structure of the thesis ................................................. 9 Chapter 1. Introduction. Objectives and structure of the thesis .................................................... 10 1.1. Introduction: Why valorise the residues of the olive sector? ........................................... 10 1.2. Objectives of this thesis ...................................................................................................... 12 1.3. Thesis structure ..................................................................................................................... 13 Chapter 2. The olive oil sector in Europe and in Spain................................................................ 15 2.1. The olive oil sector in Europe and in Spain ..................................................................... 16 2.2. Olive oil production methods ............................................................................................ 19 2.2.1. Olive presses ...................................................................................................................... -

The Effect of Low Temperature on Physiological, Biochemical And
sustainability Article The Effect of Low Temperature on Physiological, Biochemical and Flowering Functions of Olive Tree in Relation to Genotype 1, 2, 3 3 Niki Mougiou y , Boushra Baalbaki y, Georgios Doupis , Nektarios Kavroulakis , Stylianos Poulios 1, Konstantinos E. Vlachonasios 1 and Georgios C. Koubouris 3,* 1 Department of Botany, School of Biology, Faculty of Sciences, Aristotle University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] (N.M.); [email protected] (S.P.); [email protected] (K.E.V.) 2 Department of Sustainable Agriculture, Mediterranean Agronomic Institute of Chania (CIHEAM), Alsyllio Agrokepiou, P.O. Box 85, 73100 Chania, Greece; [email protected] 3 Institute for Olive Tree, Subtropical Crops and Viticulture, ELGO DEMETER, Agrokipio, 73100 Chania, Greece; [email protected] (G.D.); [email protected] (N.K.) * Correspondence: [email protected]; Tel.: +30-28210-83434 These authors contributed equally to this work. y Received: 11 October 2020; Accepted: 30 November 2020; Published: 2 December 2020 Abstract: Olive tree growth and reproduction are severely affected by temperature extremes, compromising fruit yield. In that aspect, the olive varieties “Koroneiki” and “Mastoidis” were employed in a mild cold stress experiment, imitating night frost incidents to assess their biochemical, physiological and reproductive functions in relation to genotype. The physiological performance of the stressed plants was not significantly altered, suggesting that both cultivars were well adapted to mild cold night stress. The biochemical response of the plants, regarding antioxidant enzymes, H2O2 and TBARS accumulation, confirmed that both cultivars could cope with the stress applied. The mRNA levels of the PPO gene, which participates in hydroxytyrosol biosynthesis and plant defense, were elevated after 24-h stress at 0 ◦C, in both cultivars with “Mastoidis” plants exhibiting higher levels for a longer period. -

Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium Dahliae in Olive Progenies
Article Assessment of Maternal Effects and Genetic Variability in Resistance to Verticillium dahliae in Olive Progenies Pedro Valverde Caballero, Carlos Trapero Ramírez, Diego Barranco Navero, Francisco J. López-Escudero, Ana Gordon Bermúdez-Coronel and Concepción Muñoz Díez * Department of Agronomy (Excellence Unit ‘María de Maeztu’ 2020-23), ETSIAM, University of Córdoba, 14071 Córdoba, Spain; [email protected] (P.V.C.); [email protected] (C.T.R.); [email protected] (D.B.N.); [email protected] (F.J.L.-E.); [email protected] (A.G.B.-C.) * Correspondence: [email protected] Abstract: The use of genetic resistance is likely the most efficient, economically convenient and en- vironmentally friendly control method for plant diseases, as well as a fundamental piece in an inte- grated management strategy. This is particularly important for woody crops affected by diseases in which mainly horizontal resistance mechanisms are operative, such as Verticillium wilt, caused by Verticillium dahliae. In this study, we analyzed the variability in resistance to Verticillium wilt of olive trees in progenies from five crosses: ‘Picual’ × ‘Frantoio’, ‘Arbosana’ × ‘Koroneiki’, ‘Sikitita’ × Citation: Valverde, P.; Trapero, C.; ‘Arbosana’, ‘Arbosana’ × ‘Frantoio’ and ‘Arbosana’ × ‘Arbequina’ and their respective reciprocal Barranco, D.; López-Escudero, F.J.; crosses. Additionally, seedlings of ‘Picual’ and ‘Frantoio’ in open pollination were used as controls. Gordon, A.; Díez C. M. Assessment In October 2016 and 2018, the fruits were harvested, and seeds germinated. Six-week-old seedlings of maternal effect and genetic varia- were inoculated by dipping their bare roots in a conidial suspension of V. dahliae, and disease pro- bility in resistance to Verticillium gress in terms of symptom severity and mortality was evaluated weekly. -

1 Supplimentary Material Supplier Origin Year Cultivar 1
Supplimentary Material Table S1. The cultivars used in this study and their country of origin. Supplier Origin Year Cultivar 1. Itesori Italy 2014 Nocellara 2. Frantoio di Santa Tea Italy (Firenze) 2015 Frantoio 3. Frantoio di santa Tea Italy 2015 Leccino 4. Glacomo grassi Italy 2015 Olivobianco 5. Pendolino Italy 2015 Pendolino 6. Caravella finefood Italy (Calabria) 2016 Carolea 7. Caravella finefood Italy (Puglia) 2016 Ogliarola Bio 8. Caravella finefood Italy (Puglia) 2016 Peranzana 9. Corona delle puglie Italy 2016 Coratina 10. Frantoi cutrera Italy 2016 Cerasuola 11. Frantoio di santa Tea Italy (Firenze) 2016 Moraiolo 12. Glacomo grassi Italy 2016 Leccio del Corno 13. La selvotta Italy (Abruzzo) 2016 I-77 14. Mamma regina Italy 2016 Tortiglione 15. Roi Italy 2016 Taggiasca 16. Ursini Italy 2016 Gentile di Chieti 17. Frantoi cutrera Sicily 2015 Tonda Iblea 18. Frantoi cutrera Sicily 2016 Nocellara Etnea 19. Frantoi cutrera Sicily 2016 Moresca 20. Frantoi cutrera Sicily 2016 Biancolilla 21. Frantoi cutrera Sicily 2016 Nocellara del Belice 22. Frantoi cutrera Sicily 2016 Tonda Iblea 23. Frantoi cutrera Sicily 2016 Cerasuola 24. Arbequina Spain 2016 Arbequina 25. Hojiblanca Spain 2016 Hojiblanca 26. Casas hualdo Spain 2016 Picual 27. Pago de baldios san carlos Spain 2016 Arbequina 28. Château d’estoublon France 2015 Béruguette 29. Château d’estoublon France 2015 Picholine 30. Château d’estoublon France 2015 Grossane 31. Manianis Greece 2016 Koroneiki 32. Moria ella Greece 2016 Koroneiki 33. Sam Cremona Malta 2013 Malti 34. Sam Cremona Malta 2014 Bidni 35. Sam Cremona Malta 2014 Bidni 36. Sam Cremona Malta 2014 Malti 37. Parent Siggiewi Press Malta 2014 Carolea 38. -
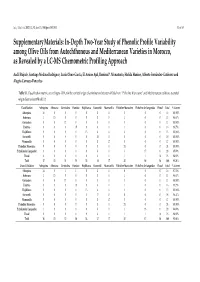
In-Depth Two-Year Study of Phenolic Profile Variability Among
Int. J. Mol. Sci. 2017, 18, 52; doi:10.3390/ijms18010052 S1 of S3 Supplementary Materials: In-Depth Two-Year Study of Phenolic Profile Variability among Olive Oils from Autochthonous and Mediterranean Varieties in Morocco, as Revealed by a LC-MS Chemometric Profiling Approach Aadil Bajoub, Santiago Medina-Rodríguez, Lucía Olmo-García, El Amine Ajal, Romina P. Monasterio, Hafida Hanine, Alberto Fernández-Gutiérrez and Alegría Carrasco-Pancorbo Table S1. Classification matrix, according to LDA, for the varietal origin discrimination between VOOs from “Picholine Marocaine” and Mediterranean cultivars (varietal origin discriminant Model 1). Classification Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 16 0 0 0 0 0 0 0 0 0 16 100.00% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 0 18 0 0 0 0 18 100.00% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 0 0 0 0 0 0 0 3 17 0 20 85.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 17 13 11 15 13 18 17 30 18 16 168 95.24% Cross-Validation Arbequina Arbosana Cornicabra Frantoio Hojiblanca Koroneiki Manzanilla Picholine Marocaine Picholine de Languedoc Picual Total % Correct Arbequina 14 0 1 1 0 0 0 0 0 0 16 87.50% Arbosana 1 13 0 0 0 0 0 1 0 0 15 86.67% Cornicabra 0 0 11 0 0 0 0 0 0 0 11 100.00% Frantoio 0 0 0 15 0 0 0 1 0 0 16 93.75% Hojiblanca 0 0 0 0 13 0 0 0 0 0 13 100.00% Koroneiki 0 0 0 0 1 17 0 0 0 0 18 94.44% Manzanilla 0 0 0 0 0 0 17 0 0 0 17 100.00% Picholine Marocaine 0 0 0 0 0 0 0 24 0 0 24 100.00% Picholine de Languedoc 1 0 0 0 0 0 0 3 16 0 20 80.00% Picual 0 0 0 0 0 0 0 1 1 16 18 88.89% Total 16 13 12 16 14 17 17 30 17 16 168 92.86% Int. -

Results ATHENA 2019
Αthena InternationalΧΑΛΚΙΝΑ ΜΕΤΑΛΛΙΑ*Olive Oil Competition OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY NAFPLIONREGION PROVINCE WEBSITE FLAVOURED ΒΙΟ 18–20 March 2019 ΜEDALS & SPECIAL PRIZES Final Participation and Awards Results DOUBLE GOLD 2019 DOUBLE GOLD MEDALS OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY REGION PROVINCE WEBSITE FLAVOURED ΒΙΟ One & Olive One & Olive Koroneiki Greece Peloponnese, Messinia Manesi www.oneolive.gr No Conde de Mirasol Aceites Mirasol Hojiblanca Spain Andalusia Córdoba www.condedemirasol.com No Palacio de Los Olivos Olivapalacios Picual Spain Castilla-La Mancha Ciudad Real www.olivapalacios.es No 60% Picual, Oro Del Desierto Coupage Rafael Alonso Aguilera Spain Andalusia Almeria www.orodeldesierto.com Yes 40% Hojiblanca Picualia Picualia Picual Spain Andalusia Jaén www.picualia.com No Horta Real Olive Gallery Picual Spain Castilla-La Mancha Toledo www.olivegallery.es No Aprutino Pescarese San- Azienda Agricola Sandro 90% Dritta, Italy Abruzzo Pescara No dro di Giacomo di Giacomo 10% Intosso 80% Hojiblanco, Venta del Barón Muela Olives Spain Andalusia Córdoba www.mueloliva.es No 20% Picudo GOLD 2019 GOLD MEDALS OLIVE OIL PRODUCER VARIETAL MAKE-UP COUNTRY REGION PROVINCE WEBSITE FLAVOURED ΒΙΟ Valdenvero Hojiblanco Colival Hojiblanca Spain Castilla-La Mancha Ciudad Real www.colival.com No Hispasur Gold Knolive Oils Picual Spain Andalusia Córdoba www.knolive.com No Aceitera Peninsular 50% Picuda, Olíria Coupage Spain Andalusia Córdoba www.aceiterapeninsular.com No Española 50% Hojiblanca Safir Basil Herbes de -

Roland Verhé
MinorMinor ComponentsComponents asas markersmarkers ofof oliveolive oiloil authenticityauthenticity andand qualityquality V. Van Hoed, R. Verhé, M. Andjelkovic Ghent University - Faculty of Bioscience Engineering - Department of Organic Chemistry [email protected] OverviewOverview I. Importance of analysis and authentification of olive oil II. Methods for determination of 1. Quality 2. Genuineness III. Authentication issues IV. Olive oil components used in this study V. Examples 1. Harvest time 2. Cultivar and geographic origin VI. Conclusions Importance of Analysis and Authentication of olive oils EVOO : mechanical extraction : unique composition and delicate aroma Lampante OO : needs refining Consumers preference: high quality + unchanged aroma and natural elements Higher price than other vegetable oils Danger of adulteration with cheaper ingredients = economical fraud = risk for consumers’ health Importance of Analysis and Authentication of olive oils Detect adulteration with cheaper ingredients Need for analysis and authentication methods Codex Alimentarius CA Commission Draft, 2003 European Commission (EC) EC Reg No 2568/1991 and amendment 1989/2003 International Olive Oil Council (IOOC) IOOC Trade Standards, 2003 = Official methods BUT sophisticated refining/adulteration Need for continual research = new methods, not (yet) evaluated by standardizing bodies, but proposed by researchers Methods for olive oil quality verification a) Methods included in the international Standards • Olive oil sampling and laboratory sample -

Preliminary Investigation of Different Drying Systems to Preserve Hydroxytyrosol and Its Derivatives in Olive Oil Filter Cake Pressurized Liquid Extracts
foods Article Preliminary Investigation of Different Drying Systems to Preserve Hydroxytyrosol and Its Derivatives in Olive Oil Filter Cake Pressurized Liquid Extracts Lucía López-Salas 1, Inés Cea 2,3, Isabel Borrás-Linares 4,*, Tatiana Emanuelli 5 , Paz Robert 2, Antonio Segura-Carretero 4,6,† and Jesús Lozano-Sánchez 1,4,† 1 Department of Food Science and Nutrition, University of Granada, Campus Universitario S/N, 18071 Granada, Spain; [email protected] (L.L.-S.); [email protected] (J.L.-S.) 2 Departamento de Ciencia de los Alimentos y Tecnología Química, Facultad de Ciencias Químicas y Farmacéuticas, Universidad de Chile, Casilla 133, Santiago 8380494, Chile; [email protected] (I.C.); [email protected] (P.R.) 3 Center for Systems Biotechnology, Fraunhofer Chile Research, Av. Del Cóndor 844 Floor 3, Santiago 8580704, Chile 4 Functional Food Research and Development Centre (CIDAF), Health Sciencie Technological Park, Avda. Del Conocimiento S/N, 18016 Granada, Spain; [email protected] 5 Department of Food Technology and Science, Center of Rural Sciences, Federal University of Santa Maria, Camobi, Santa Maria 97105-900, RS, Brazil; [email protected] 6 Citation: López-Salas, L.; Cea, I.; Department of Analytical Chemistry, Faculty of Sciences, University of Granada, 18071 Granada, Spain * Correspondence: [email protected]; Tel.: +34-9586-37083 Borrás-Linares, I.; Emanuelli, T.; † These authors are joint senior authors on this work. Robert, P.; Segura-Carretero, A.; Lozano-Sánchez, J. Preliminary Investigation of Different Drying Abstract: Phenolic compounds present in extra virgin olive oil (EVOO) could be retained in its Systems to Preserve Hydroxytyrosol byproducts during processing. -

Determinant Factors in Olive Oil Accumulation for Optimizing Harvest Time in a Context of Climate Change
EGU21-14278 https://doi.org/10.5194/egusphere-egu21-14278 EGU General Assembly 2021 © Author(s) 2021. This work is distributed under the Creative Commons Attribution 4.0 License. Determinant factors in olive oil accumulation for optimizing harvest time in a context of climate change José M. Cabezas, Estrella Muñoz, Raúl De la Rosa, Lorenzo León, and Ignacio J. Lorite ([email protected]) Olive is a woody crop extended over 10 Mha around the world (FAOSTAT, 2019), being Spain the country with the largest area (2.7 Mha). Andalusia is located in the South of Spain, with 1.6 Mha cultivated with olive trees, most of them (around 90%) dedicated to olive oil production (MAPA, 2020). This region is characterized by a great diversity of weather conditions. This diversity greatly affects important agronomic parameters of olive as the pattern of oil accumulation. This influence is different depending on the cultivar considered. In addition, this pattern of oil accumulation is a key aspect since is the most relevant trait determining the optimal harvest time. For that reason, in the present study, the relative influence of cultivar and environment, and their interaction, have been evaluated for the full pattern of oil accumulation. This study was carried out in four locations of Andalusia covering a wide range of weather conditions, and where olive trees are well established or under expansion: Antequera (Málaga), Córdoba, Úbeda (Jaén) and Gibraleón (Huelva). In 2008, five cultivars were planted in a randomized complete block design consisting in four blocks and four trees per elementary plot: Arbequina, Hojiblanca, Koroneiki, Picual and Sikitita-3 (a new registered cultivar from the olive breeding program developed by the University of Córdoba and IFAPA). -

Cytoplasmic Male Sterility in the Olive (Olea Europaea L.)
Theor Appl Genet (2000) 100:1018–1024 © Springer-Verlag 2000 ORIGINAL ARTICLE G. Besnard · B. Khadari · P. Villemur · A. Bervillé Cytoplasmic male sterility in the olive (Olea europaea L.) Received: 26 July 1999 / Accepted: 27 August 1999 Abstract The olive tree is usually hermaphrodite but Introduction self-incompatible. In the Western Mediterranean some cultivars are totally male-sterile. Three different male- In flowering plants two types of male sterility are distin- sterile phenotypes have been recognised. To infer the ge- guished according to their mode of inheritance: nuclear netic basis of male sterility we studied its inheritance and or genic male sterility (gMS) and cytoplasmic male ste- cytoplasmic diversity in wild (oleaster) and cultivated rility (CMS) (reviewed by Kaul 1988). In most cases, Mediterranean olive. In the cross Olivière×Arbequina, gMS is determined by a single locus and due to a reces- the male-sterile trait was maternally inherited and affect- sive allele. The mode of inheritance of male sterility can ed all progenies. We also checked that both chloroplast be established through crosses and back-crosses. In the and mitochondrial DNAs are maternally inherited. RFLP case of CMS, the male sterility is inherited through the studies on chloroplast and mitochondrial DNAs revealed female parent. This form of male sterility is usually ob- several cytotypes: two chlorotypes and four mitotypes in served among back-crosses and has been shown to be as- cultivars and oleaster (wild or feral Mediterranean ol- sociated with mitochondrial DNA rearrangements (Vedel ive). Furthermore, a total linkage desequilibrium be- et al. 1994). For CMS, fertility is usually regained by a tween the CCK chlorotype and the MCK mitotype in dominant nuclear restorer allele able to override the ef- cultivars and oleaster from different regions supports the fects of the cytoplasm. -

C E R T I F I C a T E
C E R T I F I C A T E Page 1 of 1 | Cert.-No.: C-04-39-16-11 /ADD /CRR Office (Germany): HALAL CONTROL, D-65428 Ruesselsheim, Stahlstr. 44, Tel.: +49 6142 301987-0, Fax.: -29, http://www.halalcontrol.eu, e-mail: [email protected] ﺣﻼﻝ ﻛﻧﺗﺭﻭﻝ HALAL CONTROL ﺷﺭﻛﺔ ﻟﻣﺭﺍﻗﺑﺔ ﻭﺗﺭﺧﻳﺹ ﺍﻟﺣﻼﻝ (Inspection and Certification Body (Germany ﺗﺷﻬﺩ ﺍﻟﺷﺭﻛﺔ ﺑﺄﻥ ﺍﻟﻣﻧﺗﺟﺎﺕ ﺃﺩﻧﺎﻩ :hereby certifies, that the products Pos Product Pos Product Pos Product 001 BORGES EXTRA VIRGIN OLIVE OIL 012 BORGES REFINED SUNFLOWER OIL HIGH OLEIC 023 ITLV REFINED GRAPESEED OIL 002 BORGES ARBEQUINA EXTRA VIRGIN OLIVE OIL 013 BORGES REFINED GRAPESEED OIL 024 ITLV EXTRA VIRGIN OLIVE OIL 003 BORGES PICUAL EXTRA VIRGIN OLIVE OIL 014 BORGES REFINED RAPESEED OIL 025 ITLV OLIVE OIL 004 BORGES HOJIBLANCA EXTRA VIRGIN OLIVE OIL 015 VIRGIN OLIVE OIL 026 ITLV ORGANIC EXTRA VIRGIN OLIVE OIL 005 ECONATURA ORGANIC FARMING EXTRA VIRGIN OLIVE OIL 016 PACIFIC CHOICE EXTRA VIRGIN OLIVE OIL 027 ITLV ARBEQUINA EXTRA VIRGIN OLIVE OIL 006 BORGES FAMILY RESERVE EXTRA VIRGIN OLIVE OIL 017 TRAMIER EXTRA VIRGIN OLIVE OIL 028 ITLV HOJIBLANCA EXTRA VIRGIN OLIVE OIL 007 BORGES OLIVE OIL 018 CESAR EXTRA VIRGIN OLIVE OIL 029 H&GSO Bakery 008 BORGES EXTRA LIGHT OLIVE OIL 019 CESAR OLIVE OIL 030 H&GSO Bakery with butter flavour 009 BORGES OLIVE OIL WITH GARLIC 020 LA RENA EXTRA VIRGIN OLIVE OIL 031 REFINED POMACE OLIVE OIL 010 BORGES REFINED OLIVE OIL 021 SUNFLOWER CRUDE OIL 032 BORGES POMACE OLIVE OIL 011 BORGES REFINED SUNFLOWER OIL 022 HIGH OLEIC SUNFLOWER CRUDE OIL --- --- ﺍﻟﻣﺻﻧﻌﺔ ﻓﻲ manufactured by BORGES AGRICULTURAL AND INDUSTRIAL EDIBLE OILS SAU ﻓﻲ (at the production site E- 25300 TÀRREGA (LLEIDA / SPAIN ﺗﺣﻘﻖ ﻣﻌﺎﻳﻳﺭ ﺍﻟﺣﻼﻝ .are Halal A HALAL CONTROL conformity assessment, documented in an audit report, has verified that the above listed products are complying with the Halal requirements in accordance with the Islamic Law. -

Olive Freeze Damage Review and Olive Knot Control
Olive Freeze Damage Review and Olive Knot Control Bill Krueger , UC farm Advisor Glenn County 1990 Freeze 2010 Freeze Critical Temperatures • Vary depending on climatic conditions • At or below 20 degrees F are often critical Factors Effecting Freeze Severity • Cold temperatures and the duration of freezing • Acclimatization • Variety • Tree age • Irrigation • Time of Pruning • Previous Crop load Low temperatures recorded in California olive producing regions, 1913-1990 Freezing event 2010 Dec. 6 through 10th Lows low 20s to mid teens 3 of 4 nights Moderate winds 3-4 mph Dec 7- higher temperatures (mid 20s or higher) with higher winds (8-10 mph) Minimum Temperatures Dec 6-10, 2010 • Arbuckle, Nickels 23.6 • Willows-west 21.2 • Artois 16.8 • Orland East 17.5 • Orland Buttes 1 23 • Orland Buttes 2 17 • Orland CIMIS Rd 25 and N 18.6 • Durham CIMIS 18 • Chico CSU Farm 19.5 • Gerber CIMIS 18.4 Number and frequency ( %) of years reporting at least one occurrence of minimum temperatures below given temperatures thresholds in olive producing areas in California 1913-1990 Continuing defoliation of Manzanillo Leaf symptoms from the 1990 freeze, spotty mottled roughened and chlorotic leaves Internal empty spaces caused by splitting apart of cells in the area of the vascular bundles Cross section of healed over Bark split in Manzanillo bark split Acclimatization • Hardiness increases when trees are exposed to cold temperatures as autumn proceeds into winter • 1985 Manzanillo block in Artois – 22 degrees in Nov. – Defoliation, bark split, olive knot