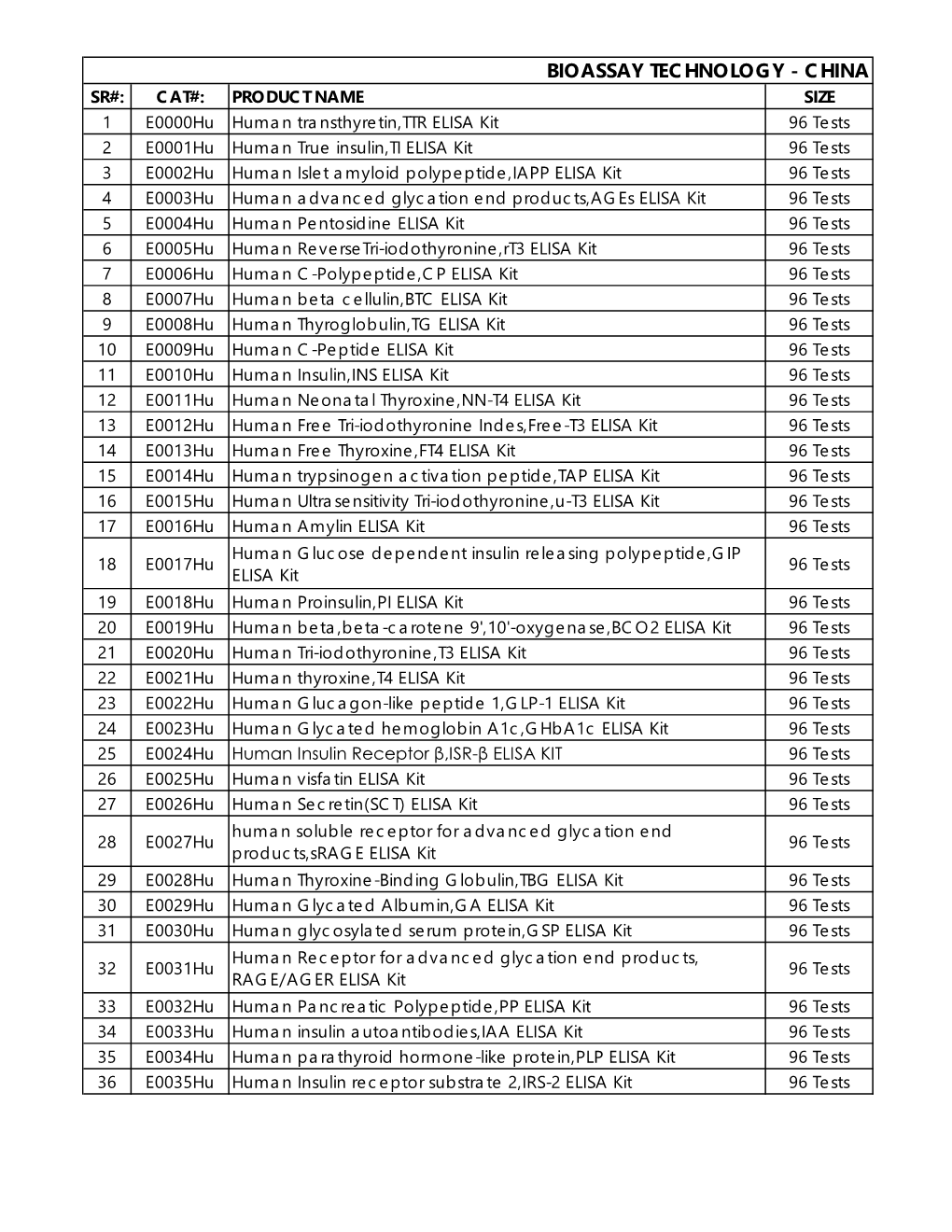
Bioassay Technology
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Environmental Influences on Endothelial Gene Expression
ENDOTHELIAL CELL GENE EXPRESSION John Matthew Jeff Herbert Supervisors: Prof. Roy Bicknell and Dr. Victoria Heath PhD thesis University of Birmingham August 2012 University of Birmingham Research Archive e-theses repository This unpublished thesis/dissertation is copyright of the author and/or third parties. The intellectual property rights of the author or third parties in respect of this work are as defined by The Copyright Designs and Patents Act 1988 or as modified by any successor legislation. Any use made of information contained in this thesis/dissertation must be in accordance with that legislation and must be properly acknowledged. Further distribution or reproduction in any format is prohibited without the permission of the copyright holder. ABSTRACT Tumour angiogenesis is a vital process in the pathology of tumour development and metastasis. Targeting markers of tumour endothelium provide a means of targeted destruction of a tumours oxygen and nutrient supply via destruction of tumour vasculature, which in turn ultimately leads to beneficial consequences to patients. Although current anti -angiogenic and vascular targeting strategies help patients, more potently in combination with chemo therapy, there is still a need for more tumour endothelial marker discoveries as current treatments have cardiovascular and other side effects. For the first time, the analyses of in-vivo biotinylation of an embryonic system is performed to obtain putative vascular targets. Also for the first time, deep sequencing is applied to freshly isolated tumour and normal endothelial cells from lung, colon and bladder tissues for the identification of pan-vascular-targets. Integration of the proteomic, deep sequencing, public cDNA libraries and microarrays, delivers 5,892 putative vascular targets to the science community. -

Investigating the Genetic Basis of Cisplatin-Induced Ototoxicity in Adult South African Patients
--------------------------------------------------------------------------- Investigating the genetic basis of cisplatin-induced ototoxicity in adult South African patients --------------------------------------------------------------------------- by Timothy Francis Spracklen SPRTIM002 SUBMITTED TO THE UNIVERSITY OF CAPE TOWN In fulfilment of the requirements for the degree MSc(Med) Faculty of Health Sciences UNIVERSITY OF CAPE TOWN University18 December of Cape 2015 Town Supervisor: Prof. Rajkumar S Ramesar Co-supervisor: Ms A Alvera Vorster Division of Human Genetics, Department of Pathology, University of Cape Town 1 The copyright of this thesis vests in the author. No quotation from it or information derived from it is to be published without full acknowledgement of the source. The thesis is to be used for private study or non- commercial research purposes only. Published by the University of Cape Town (UCT) in terms of the non-exclusive license granted to UCT by the author. University of Cape Town Declaration I, Timothy Spracklen, hereby declare that the work on which this dissertation/thesis is based is my original work (except where acknowledgements indicate otherwise) and that neither the whole work nor any part of it has been, is being, or is to be submitted for another degree in this or any other university. I empower the university to reproduce for the purpose of research either the whole or any portion of the contents in any manner whatsoever. Signature: Date: 18 December 2015 ' 2 Contents Abbreviations ………………………………………………………………………………….. 1 List of figures …………………………………………………………………………………... 6 List of tables ………………………………………………………………………………….... 7 Abstract ………………………………………………………………………………………… 10 1. Introduction …………………………………………………………………………………. 11 1.1 Cancer …………………………………………………………………………….. 11 1.2 Adverse drug reactions ………………………………………………………….. 12 1.3 Cisplatin …………………………………………………………………………… 12 1.3.1 Cisplatin’s mechanism of action ……………………………………………… 13 1.3.2 Adverse reactions to cisplatin therapy ………………………………………. -

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -

4-6 Weeks Old Female C57BL/6 Mice Obtained from Jackson Labs Were Used for Cell Isolation
Methods Mice: 4-6 weeks old female C57BL/6 mice obtained from Jackson labs were used for cell isolation. Female Foxp3-IRES-GFP reporter mice (1), backcrossed to B6/C57 background for 10 generations, were used for the isolation of naïve CD4 and naïve CD8 cells for the RNAseq experiments. The mice were housed in pathogen-free animal facility in the La Jolla Institute for Allergy and Immunology and were used according to protocols approved by the Institutional Animal Care and use Committee. Preparation of cells: Subsets of thymocytes were isolated by cell sorting as previously described (2), after cell surface staining using CD4 (GK1.5), CD8 (53-6.7), CD3ε (145- 2C11), CD24 (M1/69) (all from Biolegend). DP cells: CD4+CD8 int/hi; CD4 SP cells: CD4CD3 hi, CD24 int/lo; CD8 SP cells: CD8 int/hi CD4 CD3 hi, CD24 int/lo (Fig S2). Peripheral subsets were isolated after pooling spleen and lymph nodes. T cells were enriched by negative isolation using Dynabeads (Dynabeads untouched mouse T cells, 11413D, Invitrogen). After surface staining for CD4 (GK1.5), CD8 (53-6.7), CD62L (MEL-14), CD25 (PC61) and CD44 (IM7), naïve CD4+CD62L hiCD25-CD44lo and naïve CD8+CD62L hiCD25-CD44lo were obtained by sorting (BD FACS Aria). Additionally, for the RNAseq experiments, CD4 and CD8 naïve cells were isolated by sorting T cells from the Foxp3- IRES-GFP mice: CD4+CD62LhiCD25–CD44lo GFP(FOXP3)– and CD8+CD62LhiCD25– CD44lo GFP(FOXP3)– (antibodies were from Biolegend). In some cases, naïve CD4 cells were cultured in vitro under Th1 or Th2 polarizing conditions (3, 4). -

Regulation of Osteoblast Differentiation by Cytokine Networks
International Journal of Molecular Sciences Review Regulation of Osteoblast Differentiation by Cytokine Networks Dulshara Sachini Amarasekara 1, Sumi Kim 2 and Jaerang Rho 2,* 1 Department of Zoology and Environment Sciences, Faculty of Science, University of Colombo, Colombo 00300, Sri Lanka; [email protected] 2 Department of Microbiology and Molecular Biology, Chungnam National University, Daejeon 34134, Korea; [email protected] * Correspondence: [email protected]; Tel.: +82-42-821-6420; Fax: +82-42-822-7367 Abstract: Osteoblasts, which are bone-forming cells, play pivotal roles in bone modeling and remod- eling. Osteoblast differentiation, also known as osteoblastogenesis, is orchestrated by transcription factors, such as runt-related transcription factor 1/2, osterix, activating transcription factor 4, special AT-rich sequence-binding protein 2 and activator protein-1. Osteoblastogenesis is regulated by a network of cytokines under physiological and pathophysiological conditions. Osteoblastogenic cytokines, such as interleukin-10 (IL-10), IL-11, IL-18, interferon-γ (IFN-γ), cardiotrophin-1 and oncostatin M, promote osteoblastogenesis, whereas anti-osteoblastogenic cytokines, such as tumor necrosis factor-α (TNF-α), TNF-β, IL-1α, IL-4, IL-7, IL-12, IL-13, IL-23, IFN-α, IFN-β, leukemia inhibitory factor, cardiotrophin-like cytokine, and ciliary neurotrophic factor, downregulate os- teoblastogenesis. Although there are gaps in the body of knowledge regarding the interplay of cytokine networks in osteoblastogenesis, cytokines appear to be potential therapeutic targets in bone- related diseases. Thus, in this study, we review and discuss our osteoblast, osteoblast differentiation, osteoblastogenesis, cytokines, signaling pathway of cytokine networks in osteoblastogenesis. Keywords: osteoblast; osteoblast differentiation; osteoblastogenesis; cytokine; signaling pathway Citation: Amarasekara, D.S.; Kim, S.; Rho, J. -

Cellular and Molecular Signatures in the Disease Tissue of Early
Cellular and Molecular Signatures in the Disease Tissue of Early Rheumatoid Arthritis Stratify Clinical Response to csDMARD-Therapy and Predict Radiographic Progression Frances Humby1,* Myles Lewis1,* Nandhini Ramamoorthi2, Jason Hackney3, Michael Barnes1, Michele Bombardieri1, Francesca Setiadi2, Stephen Kelly1, Fabiola Bene1, Maria di Cicco1, Sudeh Riahi1, Vidalba Rocher-Ros1, Nora Ng1, Ilias Lazorou1, Rebecca E. Hands1, Desiree van der Heijde4, Robert Landewé5, Annette van der Helm-van Mil4, Alberto Cauli6, Iain B. McInnes7, Christopher D. Buckley8, Ernest Choy9, Peter Taylor10, Michael J. Townsend2 & Costantino Pitzalis1 1Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK. Departments of 2Biomarker Discovery OMNI, 3Bioinformatics and Computational Biology, Genentech Research and Early Development, South San Francisco, California 94080 USA 4Department of Rheumatology, Leiden University Medical Center, The Netherlands 5Department of Clinical Immunology & Rheumatology, Amsterdam Rheumatology & Immunology Center, Amsterdam, The Netherlands 6Rheumatology Unit, Department of Medical Sciences, Policlinico of the University of Cagliari, Cagliari, Italy 7Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G12 8TA, UK 8Rheumatology Research Group, Institute of Inflammation and Ageing (IIA), University of Birmingham, Birmingham B15 2WB, UK 9Institute of -

Angiopoietin/Tek Interactions Regulate MMP-9 Expression and Retinal Neovascularization
0023-6837/03/8311-1637$03.00/0 LABORATORY INVESTIGATION Vol. 83, No. 11, p. 1637, 2003 Copyright © 2003 by The United States and Canadian Academy of Pathology, Inc. Printed in U.S.A. Angiopoietin/Tek Interactions Regulate MMP-9 Expression and Retinal Neovascularization Arup Das, William Fanslow, Douglas Cerretti, Erin Warren, Nicholas Talarico, and Paul McGuire Department of Surgery (AD, PM), Division of Ophthalmology, Cell Biology and Physiology (AD, EW, NT, PM), University of New Mexico School of Medicine, and New Mexico Veterans Health Care System (AD), Albuquerque, New Mexico; and Cancer Pharmacology Amgen Washington (WF, DC), Seattle, Washington SUMMARY: The objective of the study was to determine the role of the angiopoietins in the regulation of gelatinase expression during angiogenesis, and whether inhibition of the angiopoietin/Tek interaction in vivo can suppress the extent of retinal neovascularization. Retinal microvascular endothelial cells were treated with angiopoietins and examined for the production of gelatinases. The effects of inhibiting angiopoietin binding to the Tie-2 receptor was studied in newborn mice with experimentally induced retinal neovascularization. Animals were treated with an ip injection of the Tie-2 antagonist, muTek delta Fc, while oxygen-exposed mice treated with similar concentrations of murine IgG were used as controls. The effect of muTek delta Fc on the gelatinase expression in the retina was examined by real-time RT-PCR analysis. The stimulation of cultured retinal endothelial cells with Ang-1 and -2 resulted in the increased expression of matrix metalloproteinase (MMP)-9. Ang-2 expression was up-regulated in experimental animals during the period of angiogenesis and was the greatest on Day 17 (the time of maximal angiogenic response). -

Appendix Table A.2.3.1 Full Table of All Chicken Proteins and Human Orthologs Pool Accession Human Human Protein Human Product Cell Angios Log2( Endo Gene Comp
Appendix table A.2.3.1 Full table of all chicken proteins and human orthologs Pool Accession Human Human Protein Human Product Cell AngioS log2( Endo Gene comp. core FC) Specific CIKL F1NWM6 KDR NP_002244 kinase insert domain receptor (a type III receptor tyrosine M 94 4 kinase) CWT Q8AYD0 CDH5 NP_001786 cadherin 5, type 2 (vascular endothelium) M 90 8.45 specific CWT Q8AYD0 CDH5 NP_001786 cadherin 5, type 2 (vascular endothelium) M 90 8.45 specific CIKL F1P1Y9 CDH5 NP_001786 cadherin 5, type 2 (vascular endothelium) M 90 8.45 specific CIKL F1P1Y9 CDH5 NP_001786 cadherin 5, type 2 (vascular endothelium) M 90 8.45 specific CIKL F1N871 FLT4 NP_891555 fms-related tyrosine kinase 4 M 86 -1.71 CWT O73739 EDNRA NP_001948 endothelin receptor type A M 81 -8 CIKL O73739 EDNRA NP_001948 endothelin receptor type A M 81 -8 CWT Q4ADW2 PROCR NP_006395 protein C receptor, endothelial M 80 -0.36 CIKL Q4ADW2 PROCR NP_006395 protein C receptor, endothelial M 80 -0.36 CIKL F1NFQ9 TEK NP_000450 TEK tyrosine kinase, endothelial M 77 7.3 specific CWT Q9DGN6 ECE1 NP_001106819 endothelin converting enzyme 1 M 74 -0.31 CIKL Q9DGN6 ECE1 NP_001106819 endothelin converting enzyme 1 M 74 -0.31 CWT F1NIF0 CA9 NP_001207 carbonic anhydrase IX I 74 CIKL F1NIF0 CA9 NP_001207 carbonic anhydrase IX I 74 CWT E1BZU7 AOC3 NP_003725 amine oxidase, copper containing 3 (vascular adhesion protein M 70 1) CIKL E1BZU7 AOC3 NP_003725 amine oxidase, copper containing 3 (vascular adhesion protein M 70 1) CWT O93419 COL18A1 NP_569712 collagen, type XVIII, alpha 1 E 70 -2.13 CIKL O93419 -

Enhancing the Thermostability and Activity of Uronate Dehydrogenase from Agrobacterium Tumefaciens LBA4404 by Semi-Rational Engi
Su et al. Bioresour. Bioprocess. (2019) 6:36 https://doi.org/10.1186/s40643-019-0267-3 RESEARCH Open Access Enhancing the thermostability and activity of uronate dehydrogenase from Agrobacterium tumefaciens LBA4404 by semi-rational engineering Hui‑Hui Su1†, Fei Peng1†, Pei Xu1, Xiao‑Ling Wu1, Min‑Hua Zong1, Ji‑Guo Yang2 and Wen‑Yong Lou1* Abstract Background: Glucaric acid, one of the aldaric acids, has been declared a “top value‑added chemical from biomass”, and is especially important in the food and pharmaceutical industries. Biocatalytic production of glucaric acid from glucuronic acid is more environmentally friendly, efcient and economical than chemical synthesis. Uronate dehydro‑ genases (UDHs) are the key enzymes for the preparation of glucaric acid in this way, but the poor thermostability and low activity of UDH limit its industrial application. Therefore, improving the thermostability and activity of UDH, for example by semi‑rational design, is a major research goal. Results: In the present work, three UDHs were obtained from diferent Agrobacterium tumefaciens strains. The three UDHs have an approximate molecular weight of 32 kDa and all contain typically conserved UDH motifs. All three UDHs showed optimal activity within a pH range of 6.0–8.5 and at a temperature of 30 °C, but the UDH from A. tume- 1 1 faciens (At) LBA4404 had a better catalytic efciency than the other two UDHs (800 vs 600 and 530 s− mM− ). To fur‑ ther boost the catalytic performance of the UDH from AtLBA4404, site‑directed mutagenesis based on semi‑rational design was carried out. An A39P/H99Y/H234K triple mutant showed a 400‑fold improvement in half‑life at 59 °C, a 10 5 °C improvement in T50 value and a 2.5‑fold improvement in specifc activity at 30 °C compared to wild‑type UDH. -

Cloud-Clone 16-17
Cloud-Clone - 2016-17 Catalog Description Pack Size Supplier Rupee(RS) ACB028Hu CLIA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 74750 AEA044Hu ELISA Kit for Anti-Growth Hormone Antibody (Anti-GHAb) 96T Cloud-Clone 74750 AEA255Hu ELISA Kit for Anti-Apolipoprotein Antibodies (AAHA) 96T Cloud-Clone 74750 AEA417Hu ELISA Kit for Anti-Proteolipid Protein 1, Myelin Antibody (Anti-PLP1) 96T Cloud-Clone 74750 AEA421Hu ELISA Kit for Anti-Myelin Oligodendrocyte Glycoprotein Antibody (Anti- 96T Cloud-Clone 74750 MOG) AEA465Hu ELISA Kit for Anti-Sperm Antibody (AsAb) 96T Cloud-Clone 74750 AEA539Hu ELISA Kit for Anti-Myelin Basic Protein Antibody (Anti-MBP) 96T Cloud-Clone 71250 AEA546Hu ELISA Kit for Anti-IgA Antibody 96T Cloud-Clone 71250 AEA601Hu ELISA Kit for Anti-Myeloperoxidase Antibody (Anti-MPO) 96T Cloud-Clone 71250 AEA747Hu ELISA Kit for Anti-Complement 1q Antibody (Anti-C1q) 96T Cloud-Clone 74750 AEA821Hu ELISA Kit for Anti-C Reactive Protein Antibody (Anti-CRP) 96T Cloud-Clone 74750 AEA895Hu ELISA Kit for Anti-Insulin Receptor Antibody (AIRA) 96T Cloud-Clone 74750 AEB028Hu ELISA Kit for Anti-Albumin Antibody (AAA) 96T Cloud-Clone 71250 AEB264Hu ELISA Kit for Insulin Autoantibody (IAA) 96T Cloud-Clone 74750 AEB480Hu ELISA Kit for Anti-Mannose Binding Lectin Antibody (Anti-MBL) 96T Cloud-Clone 88575 AED245Hu ELISA Kit for Anti-Glutamic Acid Decarboxylase Antibodies (Anti-GAD) 96T Cloud-Clone 71250 AEK505Hu ELISA Kit for Anti-Heparin/Platelet Factor 4 Antibodies (Anti-HPF4) 96T Cloud-Clone 71250 CCA005Hu CLIA Kit for Angiotensin II -

(LXR/NR1H3) Regulates Differentiation of Hepatocyte-Like Cells Via
Liver X receptor α (LXRα/NR1H3) regulates differentiation of hepatocyte-like cells via reciprocal regulation of HNF4α Kai-Ting Chen, Kelig Pernelle, Yuan-Hau Tsai, Yu-Hsuan Wu, Jui-Yu Hsieh, Ko-Hsun Liao, Christiane Guguen-Guillouzo, Hsei-Wei Wang To cite this version: Kai-Ting Chen, Kelig Pernelle, Yuan-Hau Tsai, Yu-Hsuan Wu, Jui-Yu Hsieh, et al.. Liver X recep- tor α (LXRα/NR1H3) regulates differentiation of hepatocyte-like cells via reciprocal regulation of HNF4α. Journal of Hepatology, Elsevier, 2014, 61 (6), pp.1276-1286. 10.1016/j.jhep.2014.07.025. hal-01072495 HAL Id: hal-01072495 https://hal.archives-ouvertes.fr/hal-01072495 Submitted on 8 Oct 2014 HAL is a multi-disciplinary open access L’archive ouverte pluridisciplinaire HAL, est archive for the deposit and dissemination of sci- destinée au dépôt et à la diffusion de documents entific research documents, whether they are pub- scientifiques de niveau recherche, publiés ou non, lished or not. The documents may come from émanant des établissements d’enseignement et de teaching and research institutions in France or recherche français ou étrangers, des laboratoires abroad, or from public or private research centers. publics ou privés. This is the author’s final draft post-refeering (post-print) Find more peer-reviewed articles on our open access repository: http://hal-univ-rennes1.archives-ouvertes.fr/ MANUSCRIPT ACCEPTED Liver X receptor a (LXRa/NR1H3) regulates differentiation of hepatocyte-like cells via reciprocal regulation of HNF4a Kai-Ting Chen1,2,3, Kelig Pernelle4, Yuan-Hau -

IL-37 Is Increased in Brains of Children with Autism Spectrum Disorder and Inhibits Human Microglia Stimulated by Neurotensin
IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin Irene Tsilionia, Arti B. Patela,b, Harry Pantazopoulosc,1, Sabina Berrettac, Pio Contid, Susan E. Leemane,2, and Theoharis C. Theoharidesa,b,f,2 aLaboratory of Molecular Immunopharmacology and Drug Discovery, Department of Immunology, Tufts University School of Medicine, Boston, MA 02111; bSackler School of Graduate Biomedical Sciences, Tufts University School of Medicine, Boston, MA 02111; cTranslational Neuroscience Laboratory, McLean Hospital, Harvard Medical School, Belmont, MA 02478; dImmunology Division, Postgraduate Medical School, University of Chieti, 65100 Pescara, Italy; eDepartment of Pharmacology, Boston University School of Medicine, Boston, MA 02118; and fDepartment of Internal Medicine, Tufts Medical Center, Tufts University School of Medicine, Boston, MA 02111 Contributed by Susan E. Leeman, July 15, 2019 (sent for review May 3, 2019; reviewed by Charles A. Dinarello and Richard E. Frye) Autism spectrum disorder (ASD) does not have a distinct pathogenesis to Toll-like receptor (TLR) activation. An IL-37 precursor (pro– or effective treatment. Increasing evidence supports the presence of IL-37) is cleaved by caspase-1 into mature IL-37, some of which immune dysfunction and inflammation in the brains of children with (∼20%) enters the nucleus and the rest is released along with the ASD. In this report, we present data that gene expression of the pro–IL-37 outside the cells (34) where both are biologically active. antiinflammatory cytokine IL-37, as well as of the proinflammatory Extracellular proteases can then process pro–IL-37 into a much cytokines IL-18 and TNF, is increased in the amygdala and dorsolateral more biologically active form as shown for the recombinant IL-37b prefrontal cortex of children with ASD as compared to non-ASD with the N terminus Val46 (V46-218) (35).