Regulation of Osteoblast Differentiation by Cytokine Networks
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

Protein Identities in Evs Isolated from U87-MG GBM Cells As Determined by NG LC-MS/MS
Protein identities in EVs isolated from U87-MG GBM cells as determined by NG LC-MS/MS. No. Accession Description Σ Coverage Σ# Proteins Σ# Unique Peptides Σ# Peptides Σ# PSMs # AAs MW [kDa] calc. pI 1 A8MS94 Putative golgin subfamily A member 2-like protein 5 OS=Homo sapiens PE=5 SV=2 - [GG2L5_HUMAN] 100 1 1 7 88 110 12,03704523 5,681152344 2 P60660 Myosin light polypeptide 6 OS=Homo sapiens GN=MYL6 PE=1 SV=2 - [MYL6_HUMAN] 100 3 5 17 173 151 16,91913397 4,652832031 3 Q6ZYL4 General transcription factor IIH subunit 5 OS=Homo sapiens GN=GTF2H5 PE=1 SV=1 - [TF2H5_HUMAN] 98,59 1 1 4 13 71 8,048185945 4,652832031 4 P60709 Actin, cytoplasmic 1 OS=Homo sapiens GN=ACTB PE=1 SV=1 - [ACTB_HUMAN] 97,6 5 5 35 917 375 41,70973209 5,478027344 5 P13489 Ribonuclease inhibitor OS=Homo sapiens GN=RNH1 PE=1 SV=2 - [RINI_HUMAN] 96,75 1 12 37 173 461 49,94108966 4,817871094 6 P09382 Galectin-1 OS=Homo sapiens GN=LGALS1 PE=1 SV=2 - [LEG1_HUMAN] 96,3 1 7 14 283 135 14,70620005 5,503417969 7 P60174 Triosephosphate isomerase OS=Homo sapiens GN=TPI1 PE=1 SV=3 - [TPIS_HUMAN] 95,1 3 16 25 375 286 30,77169764 5,922363281 8 P04406 Glyceraldehyde-3-phosphate dehydrogenase OS=Homo sapiens GN=GAPDH PE=1 SV=3 - [G3P_HUMAN] 94,63 2 13 31 509 335 36,03039959 8,455566406 9 Q15185 Prostaglandin E synthase 3 OS=Homo sapiens GN=PTGES3 PE=1 SV=1 - [TEBP_HUMAN] 93,13 1 5 12 74 160 18,68541938 4,538574219 10 P09417 Dihydropteridine reductase OS=Homo sapiens GN=QDPR PE=1 SV=2 - [DHPR_HUMAN] 93,03 1 1 17 69 244 25,77302971 7,371582031 11 P01911 HLA class II histocompatibility antigen, -

IL-37 Is Increased in Brains of Children with Autism Spectrum Disorder and Inhibits Human Microglia Stimulated by Neurotensin
IL-37 is increased in brains of children with autism spectrum disorder and inhibits human microglia stimulated by neurotensin Irene Tsilionia, Arti B. Patela,b, Harry Pantazopoulosc,1, Sabina Berrettac, Pio Contid, Susan E. Leemane,2, and Theoharis C. Theoharidesa,b,f,2 aLaboratory of Molecular Immunopharmacology and Drug Discovery, Department of Immunology, Tufts University School of Medicine, Boston, MA 02111; bSackler School of Graduate Biomedical Sciences, Tufts University School of Medicine, Boston, MA 02111; cTranslational Neuroscience Laboratory, McLean Hospital, Harvard Medical School, Belmont, MA 02478; dImmunology Division, Postgraduate Medical School, University of Chieti, 65100 Pescara, Italy; eDepartment of Pharmacology, Boston University School of Medicine, Boston, MA 02118; and fDepartment of Internal Medicine, Tufts Medical Center, Tufts University School of Medicine, Boston, MA 02111 Contributed by Susan E. Leeman, July 15, 2019 (sent for review May 3, 2019; reviewed by Charles A. Dinarello and Richard E. Frye) Autism spectrum disorder (ASD) does not have a distinct pathogenesis to Toll-like receptor (TLR) activation. An IL-37 precursor (pro– or effective treatment. Increasing evidence supports the presence of IL-37) is cleaved by caspase-1 into mature IL-37, some of which immune dysfunction and inflammation in the brains of children with (∼20%) enters the nucleus and the rest is released along with the ASD. In this report, we present data that gene expression of the pro–IL-37 outside the cells (34) where both are biologically active. antiinflammatory cytokine IL-37, as well as of the proinflammatory Extracellular proteases can then process pro–IL-37 into a much cytokines IL-18 and TNF, is increased in the amygdala and dorsolateral more biologically active form as shown for the recombinant IL-37b prefrontal cortex of children with ASD as compared to non-ASD with the N terminus Val46 (V46-218) (35). -
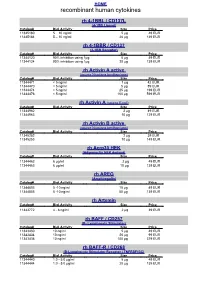
Recombinant Human Cytokines
HOME recombinant human cytokines rh 4-1BBL / CD137L (4-1BB Ligand) Catalog# Biol.Activity Size Price 11345180 5 – 10 ng/ml 5 µg 49 EUR 11345184 5 – 10 ng/ml 20 µg 139 EUR rh 4-1BBR / CD137 (4-1BB Receptor) Catalog# Biol.Activity Size Price 11344120 90% inhibition using 1µg 5 µg 49 EUR 11344124 90% inhibition using 1µg 20 µg 139 EUR rh Activin A active (source Nicotiana benthamiana) Catalog# Biol.Activity Size Price 11344471 < 5 ng/ml 1 µg 42 EUR 11344470 < 5 ng/ml 5 µg 89 EUR 11344474 < 5 ng/ml 25 µg 199 EUR 11344476 < 5 ng/ml 100 µg 599 EUR rh Activin A (source E.coli) Catalog# Biol.Activity Size Price 11344962 2 µg 49 EUR 11344963 10 µg 129 EUR rh Activin B active (source Nicotiana benthamiana) Catalog# Biol.Activity Size Price 11345252 2 µg 39 EUR 11345253 10 µg 149 EUR rh Acrp30 HEK (Adiponectin HEK derived) Catalog# Biol.Activity Size Price 11344462 6 µg/ml 2 µg 49 EUR 11344463 6 µg/ml 10 µg 139 EUR rh AREG (Amphiregulin) Catalog# Biol.Activity Size Price 11344803 5 -10 ng/ml 10 µg 49 EUR 11344805 5 -10 ng/ml 50 µg 139 EUR rh Artemin Catalog# Biol.Activity Size Price 11343772 4 - 8 ng/ml 2 µg 39 EUR rh BAFF / CD257 (B- Lymphocyte Stimulator) Catalog# Biol.Activity Size Price 11343430 10 ng/ml 5 µg 49 EUR 11343434 10 ng/ml 20 µg 99 EUR 11343436 10 ng/ml 100 µg 379 EUR rh BAFF-R / CD268 (B-Lymphocyte Stimulator Receptor / TNFRSF13C) Catalog# Biol.Activity Size Price 11344440 1.0 - 5.0 µg/ml 5 µg 49 EUR 11344444 1.0 - 5.0 µg/ml 20 µg 139 EUR HOME recombinant human cytokines rh BCA-1 (CXCL13) Catalog# Biol.Activity Size Price 11344180 -

Effects of Broccoli and Carrots on Fecal Microrna Expression in Infants: a Short-Term Feeding Study
University of Arkansas, Fayetteville ScholarWorks@UARK Theses and Dissertations 7-2020 Effects of Broccoli and Carrots on Fecal MicroRNA Expression in Infants: A Short-term Feeding Study Kaleigh E. Beane University of Arkansas, Fayetteville Follow this and additional works at: https://scholarworks.uark.edu/etd Part of the Human and Clinical Nutrition Commons, Molecular, Genetic, and Biochemical Nutrition Commons, and the Nutritional Epidemiology Commons Citation Beane, K. E. (2020). Effects of Broccoli and Carrots on Fecal MicroRNA Expression in Infants: A Short- term Feeding Study. Theses and Dissertations Retrieved from https://scholarworks.uark.edu/etd/3826 This Thesis is brought to you for free and open access by ScholarWorks@UARK. It has been accepted for inclusion in Theses and Dissertations by an authorized administrator of ScholarWorks@UARK. For more information, please contact [email protected]. Effects of Broccoli and Carrots on Fecal MicroRNA Expression in Infants: A Short-term Feeding Study A thesis submitted in partial fulfillment of the requirements for the degree of Master of Science in Human Environmental Sciences by Kaleigh E. Beane University of Arkansas Bachelor of Science in Human Nutrition and Hospitality Innovation, 2018 July 2020 University of Arkansas This thesis is approved for recommendation to the Graduate Council. _________________________________ ______________________________ Jae Kyeom Kim, Ph.D. Betsy Garrison, Ph.D. Thesis Chair Thesis Co-Chair _________________________________ _________________________________ Jiangchao Zhao, Ph.D. Sabrina Trudo, Ph.D. Committee Member Committee Member Abstract Diets, through multiple mechanisms, cause significant impact on human health and disease etiology; proposed modes of action include modulation of non-coding RNAs and influences on the gut microbiome. -

IL-37 Inhibits Lipopolysaccharide-Induced Osteoclast Formation and Bone Resorption in Vivo
View metadata, citation and similar papers at core.ac.uk brought to you by CORE IL-37 inhibits lipopolysaccharide-induced osteoclast formation and bone resorption in vivo 著者 JAFARI SAEED number 44 学位授与機関 Tohoku University 学位授与番号 歯博第748号 URL http://hdl.handle.net/10097/00097027 ジャファリ サイード 氏 名(本籍) : JAFARI SAEED 学 位 の 種 類 : 博 士 ( 歯 学 ) 学 位 記 番 号 : 歯 博 第 7 4 8 号 学位授与年月日 : 平 成 2 8 年 3 月 2 5 日 学位授与の要件 : 学位規則第4条第1項該当 研究科・専攻 : 東北大学大学院歯学研究科(博士課程)歯科学専攻 学位論文題目 : IL-37 inhibits lipopolysaccharide-induced osteoclast formation and bone resorption in vivo (Interleukin-37 の lipopolysaccharide による破骨細胞形 成及び骨吸収への影響の in vivo での検討) 論文審査委員 : (主査)教授 齋 藤 正 寛 教授 高 田 春比古 教授 山 本 照 子 論文内容要旨 IL-37 is a newly defined member of the IL-1 cytokine family. IL-37 expressed only in certain types of human organs and cells such as; testis, thymus, uterus, kidney, heart, peripheral blood mononuclear cells (PBMCs) and dendritic cells. It has been reported that IL-37 inhibited innate immunity and inflammatory respons es in autoimmune diseases and tumors. Osteoclast recruitment might be central to diseases involving bone erosion, such as rheumatoid arthritis. Osteoclasts derived from bone marrow cells regulate bone resorption and remodeling. Two factors are required for osteoclasts formation and activation: receptor activator of NF-kB ligand (RANKL) and macrophage colony stimulating factor (M-CSF). It has also been reported that tumor necrosis factor (TNF)- α induced osteoclast formation in vitro and in vivo. Lipopolysaccharide (LPS), which is a bacterial cell wall component, is known as a potent inducer of inflammation. -

Role of the Novel Cytokine IL-37 in Inflammations and Tumors of Human
Obstetrics and Gynecology Reports Review Article ISSN: 2515-2955 Role of the novel cytokine IL-37 in inflammations and tumors of human reproductive system Jingzhe Zhao1 , Lin Qiu1, Yunxin Xu1, Yien Xu1, Yanxian Guo1, Peng Li1, Zhangquan Chen2, Hongsheng Guo1 and Sen Wang1* 1Histology and embryology teaching and research section, Basic medicine department of Guangdong medical university, Guangdong province, China 2School of laboratory medicine of Guangdong medical university, Guangdong province, China Abstract IL-37 is one of the newest members of interleukin family and is also one of the most popular cytokine. It has been identified as an inhibitor of both innate and adaptive immunity in those most recently presented researches. However, the biological properties and the related mechanism of IL-37 haven’t been fully characterized. Although changes of IL-37 levels in reproductive system diseases and its role in tumors were different even potentially opposite, clues have shown that IL-37 is valuable for the diagnosis and therapy of inflammatory diseases and tumors. IL-37 plays mainly an anti-inflammatory role in Adenomyosis and Endometriosis and an anti-cancer role in cervical cancer and breast cancer. Recent controversial focuses are on the areas of tumors and ovarian diseases. In this review, the roles of IL-37 in reproductive system diseases will be presented. Introduction The expression and distribution of these five subtypes are different in human tissues and cells (Figure 1). IL-37 usually refers to IL-37b. Interleukin 37 (IL-37) is a newly discovered anti-inflammatory IL-37 shows low expressions in healthy tissues and normal cells, cytokine and was renamed in 2010 [1]. -

Interleukin-37 Monomer Is the Active Form for Reducing Innate Immunity
Interleukin-37 monomer is the active form for reducing innate immunity Elan Z. Eisenmessera,1, Adrian Gottschlichb, Jasmina S. Redzica, Natasia Paukovicha, Jay C. Nixc, Tania Azamd, Lingdi Zhanga, Rui Zhaoa, Jeffrey S. Kiefta, Erlinda Thee, Xianzhong Menge, and Charles A. Dinarellod,f,1 aDepartment of Biochemistry and Molecular Genetics, University of Colorado Denver, Aurora, CO 80238; bLudwig Maximilian University of Munich, 803337 Munich, Germany; cMolecular Biology Consortium, Advanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA 94720; dDepartment of Medicine, University of Colorado Denver, Aurora, CO 80238; eDepartment of Surgery, University of Colorado Denver, Aurora, CO 80238; and fRadboud University Medical Center, 6525 GA, Nijmegen, The Netherlands Contributed by Charles A. Dinarello, December 31, 2018 (sent for review November 26, 2018; reviewed by Martina Molgora and Magnus Wolf-Watz) Interleukin-37 (IL-37), a member of the IL-1 family of cytokines, is a 10 family members that are fully active as swapped dimers (13, fundamental suppressor of innate and acquired immunities. Here, 14) to chemokines, such as IL-8 that engage their receptors we used an integrative approach that combines biophysical, primarily as monomers (15–17). Indeed, the recent crystal biochemical, and biological studies to elucidate the unique char- structure of IL-37 by Ellisdon et al. (12) has afforded the engi- acteristics of IL-37. Our studies reveal that single amino acid neering of IL-37 mutant forms that disrupt dimer formation and mutations at the IL-37 dimer interface that result in the stable simultaneously lead to higher IL-37 activities. However, the ac- formation of IL-37 monomers also remain monomeric at high tivity of these IL-37 mutant forms were compared well below micromolar concentrations and that these monomeric IL-37 forms their determined self-association affinities where dimer forma- comprise higher antiinflammatory activities than native IL-37 on tion would be expected to be negligible, suggesting the presence multiple cell types. -

RT² Profiler PCR Array (384-Well Format) Human Inflammatory Response & Autoimmunity
RT² Profiler PCR Array (384-Well Format) Human Inflammatory Response & Autoimmunity Cat. no. 330231 PAHS-3803ZE For pathway expression analysis Format For use with the following real-time cyclers RT² Profiler PCR Array, Applied Biosystems® models 7900HT (384-well block), Format E ViiA™ 7 (384-well block); Bio-Rad CFX384™ RT² Profiler PCR Array, Roche® LightCycler® 480 (384-well block) Format G Description The Human Inflammatory Response & Autoimmunity RT² Profiler PCR Array profiles the expression of 370 key genes involved in immune response during autoimmunity and inflammation. It represents the expression of inflammatory cytokines, chemokines, and their receptors. It also contains genes related to the production and metabolism of cytokines. Genes involved in cytokine-cytokine receptor interactions are as well as thoroughly researched panels of genes involved in the acute-phase, inflammatory, and humoral immune responses. Using real-time PCR, you can easily and reliably analyze the expression of a focused panel of genes related to autoimmunity and inflammation with this array. For further details, consult the RT² Profiler PCR Array Handbook. Sample & Assay Technologies Shipping and storage RT² Profiler PCR Arrays in formats E and G are shipped at ambient temperature, on dry ice, or blue ice packs depending on destination and accompanying products. For long term storage, keep plates at –20°C. Note: Ensure that you have the correct RT² Profiler PCR Array format for your real-time cycler (see table above). Note: Open the package and store -
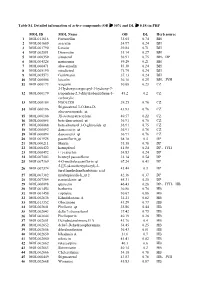
MOL ID MOL Name OB DL Herb Source 1 MOL011616 Fortunellin
Table S1. Detailed information of active compounds (OB ≥ 30% and DL ≥ 0.18) in PHF MOL ID MOL Name OB DL Herb source 1 MOL011616 Fortunellin 35.65 0.74 BH 2 MOL001689 acacetin 34.97 0.24 BH 3 MOL001790 Linarin 39.84 0.71 BH 4 MOL002881 Diosmetin 31.14 0.27 BH 5 MOL000359 sitosterol 36.91 0.75 BH、DP 6 MOL004328 naringenin 59.29 0.21 BH 7 MOL000471 aloe-emodin 83.38 0.24 BH 8 MOL005190 eriodictyol 71.79 0.24 BH 9 MOL005573 Genkwanin 37.13 0.24 BH 10 MOL000006 luteolin 36.16 0.25 BH、JYH 11 MOL000173 wogonin 30.68 0.23 CZ 2-Hydroxyisoxypropyl-3-hydroxy-7- 12 MOL000179 isopentene-2,3-dihydrobenzofuran-5- 45.2 0.2 CZ carboxylic 13 MOL000184 NSC63551 39.25 0.76 CZ Stigmasterol 3-O-beta-D- 14 MOL000186 43.83 0.76 CZ glucopyranoside_qt 15 MOL000188 3β-acetoxyatractylone 40.57 0.22 CZ 16 MOL000085 beta-daucosterol_qt 36.91 0.75 CZ 17 MOL000088 beta-sitosterol 3-O-glucoside_qt 36.91 0.75 CZ 18 MOL000092 daucosterin_qt 36.91 0.76 CZ 19 MOL000094 daucosterol_qt 36.91 0.76 CZ 20 MOL001925 paeoniflorin_qt 68.18 0.4 DP 21 MOL000211 Mairin 55.38 0.78 DP 22 MOL000422 kaempferol 41.88 0.24 DP、JYH 23 MOL000492 (+)-catechin 54.83 0.24 DP 24 MOL007003 benzoyl paeoniflorin 31.14 0.54 DP 25 MOL007369 4-O-methylpaeoniflorin_qt 67.24 0.43 DP 5-[[5-(4-methoxyphenyl)-2- 26 MOL007374 43.44 0.3 DP furyl]methylene]barbituric acid 27 MOL007382 mudanpioside-h_qt 2 42.36 0.37 DP 28 MOL007384 paeonidanin_qt 65.31 0.35 DP 29 MOL000098 quercetin 46.43 0.28 DP、JYH、HB 30 MOL001454 berberine 36.86 0.78 HB 31 MOL001458 coptisine 30.67 0.86 HB 32 MOL002636 Kihadalactone A 34.21 -

Advances in Mast Cell Activation by IL-1 and IL-33 in Sjögren's Syndrome
International Journal of Molecular Sciences Review Advances in Mast Cell Activation by IL-1 and IL-33 in Sjögren’s Syndrome: Promising Inhibitory Effect of IL-37 Pio Conti 1,* , Luisa Stellin 2, Alesssandro Caraffa 3, Carla E. Gallenga 4 , Rhiannon Ross 5, Spyros K. Kritas 6, Ilias Frydas 7, Ali Younes 8, Paolo Di Emidio 9 and Gianpaolo Ronconi 10 1 Postgraduate Medical School, University of Chieti, 66013 Chieti, Italy 2 Department of Medicine and Science of Ageing, University of Chieti, 66013 Chieti, Italy; [email protected] 3 School of Pharmacy, University of Camerino, 62032 Camerino, Italy; alecaraff[email protected] 4 Department of Biomedical Sciences and Specialist Surgery, Section of Ophthalmology, University of Ferrara, 44121 Ferrara, Italy; [email protected] 5 University of Pennsylvania School of Veterinary Medicine, Philadelphia, PA 19104, USA; [email protected] 6 Department of Microbiology, University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] 7 School of Veterinary Medicine, University of Thessaloniki, 54124 Thessaloniki, Greece; [email protected] 8 Centro Medico “Mai più Dolore”, 65100 Pescara, Italy; [email protected] 9 Maxillofacial Surgery “G. azzini” Hospital, 64100 Teramo, Italy; [email protected] 10 Fondazione Policlinico Universitario A. Gemelli IRCCS, Università Cattolica del Sacro Cuore, 00100 Roma, Italy; [email protected] * Correspondence: [email protected] Received: 13 May 2020; Accepted: 12 June 2020; Published: 16 June 2020 Abstract: Sjögren’s syndrome (SS) is a chronic autoimmune inflammatory disease that affects primarily older women and is characterized by irreversible damage of the exocrine glands, including tear (xerophthalmia) and salivary glands (xerostomia). Secretory glands lose their functionality due to the infiltration of immune cells, which produce cytokines and cause inflammation. -

The Role of Interleukin-37 in Inflammation: Suppression Or
ISSN: 2469-5726 Ding et al. J Rheum Dis Treat 2018, 4:058 DOI: 10.23937/2469-5726/1510058 Volume 4 | Issue 1 Journal of Open Access Rheumatic Diseases and Treatment MINI REVIEW The Role of Interleukin-37 in Inflammation: Suppression or Pro- motion? Liping Ding, Xiaoping Hong and Dongzhou Liu* Check for Department of Rheumatology and Immunology, Shenzhen People’s Hospital, The Second Clinical updates Medical College, Jinan University, Shenzhen, China *Corresponding author: Dongzhou Liu, Department of Rheumatology and Immunology, Shenzhen People’s Hospital, The Second Clinical Medical College, Jinan University, Shenzhen, China, E-mail: [email protected] We read with great interest the paper presented in expression of human IL-37 in mice could protect cardio- the current issue of the Journal of innate immunity by myocytes from apoptosis and suppress the migration Schauer, et al. [1] showing the pathogenic role of Inter- ability of neutrophils in myocardial ischaemia/reper- leukin-37 (IL-37) in the murine model of Streptococcus fusion injury condition [13]. Administration of IL-37 to pneumoniae pneumonia. This study found that recruit- the mice which subjected to endotoxin or high fat diet ment of alveolar macrophages and neutrophils was could attenuate aortic valve thickening and control the markedly increased in the mice transgenic for human progression of calcific aortic valve disease [14]. Previous IL-37b (IL-37tg) with heavier pneumococcal burden, re- studies have shown that IL-37 has therapeutic potential sulting in necrotizing pneumonia with augmented death for these mouse models of human disease. of infiltrating neutrophils, and leading to enhanced in- flammation, tissue damage, and mortality. -

Parsing the IL-37-Mediated Suppression of Inflammasome
cells Article Parsing the IL-37-Mediated Suppression of Inflammasome Function Ina Rudloff 1,2 , Holly K. Ung 1,2, Jennifer K. Dowling 3,4,5, Ashley Mansell 4,5, Laura D’Andrea 6, Andrew M. Ellisdon 6, James C. Whisstock 6,7, Philip J. Berger 1,2, Claudia A. Nold-Petry 1,2 and Marcel F. Nold 1,2,8,* 1 Department of Paediatrics, Monash University, Clayton, Victoria 3168, Australia; ina.rudloff@hudson.org.au (I.R.); [email protected] (H.K.U.); [email protected] (P.J.B.); [email protected] (C.A.N.-P.) 2 Ritchie Centre, Hudson Institute of Medical Research, Clayton, Victoria 3168, Australia 3 School of Pharmacy and Biomolecular Sciences, Royal College of Surgeons in Ireland, 123 St Stephens Green, Dublin 2, Ireland; [email protected] 4 Centre for Innate Immunity and Infectious Diseases, Hudson Institute of Medical Research, Clayton, Victoria 3168, Australia; [email protected] 5 Department of Molecular and Translational Sciences, Monash University, Clayton, Victoria 3168, Australia 6 Biomedicine Discovery Institute, Monash University, Clayton, Victoria 3168, Australia; [email protected] (L.D.); [email protected] (A.M.E.); [email protected] (J.C.W.) 7 Australian Research Council Centre of Excellence in Advanced Molecular Imaging, Monash University, Clayton, Victoria 3168, Australia 8 Monash Newborn, Monash Children’s Hospital, Clayton, Victoria 3168, Australia * Correspondence: [email protected]; Tel.: +61-3-8572-2815 Received: 30 November 2019; Accepted: 8 January 2020; Published: 10 January 2020 Abstract: Interleukin (IL)-37 is a member of the IL-1 family of cytokines.