Caf Drug Benefit List: Quick Reference Guide
Total Page:16
File Type:pdf, Size:1020Kb
Load more
Recommended publications
-

LGM-Pharma-Regulatory-1527671011
Pipeline Products List Specialty Portfolio Updated Q2 2018 Updated Q2 2018 See below list of newly approved API’s, samples are readily available for your R&D requirements: Inhalation Ophthalmic Transdermal Sublingual Abaloparatide Defibrotide Sodium Liraglutide Rituximab Abciximab Deforolimus Lixisenatide Rivastigmine Aclidinium Bromide Azelastine HCl Agomelatine Alprazolam Abemaciclib Delafloxacin Lumacaftor Rivastigmine Hydrogen Tartrate Beclomethasone Dipropionate Azithromycin Amlodipine Aripiprazole Acalabrutinib Denosumab Matuzumab Rizatriptan Benzoate Budesonide Besifloxacin HCl Apomorphine Eletriptan HBr Aclidinium Bromide Desmopressin Acetate Meloxicam Rocuronium Bromide Adalimumab Difluprednate Memantine Hydrochloride Rolapitant Flunisolide Bimatoprost Clonidine Epinephrine Aflibercept Dinoprost Tromethamine Micafungin Romidepsin Fluticasone Furoate Brimonidine Tartrate Dextromethorphan Ergotamine Tartrate Agomelatine Dolasetron Mesylate Mitomycin C Romosozumab Fluticasone Propionate Bromfenac Sodium Diclofenac Levocetrizine DiHCl Albiglutide Donepezil Hydrochloride Mometasone Furoate Rotigotine Formoterol Fumarate Cyclosporine Donepezil Meclizine Alectinib Dorzolamide Hydrochloride Montelukast Sodium Rucaparib Iloprost Dexamethasone Valerate Estradiol Melatonin Alemtuzumab Doxercalciferol Moxifloxacin Hydrochloride Sacubitril Alirocumab Doxorubicin Hydrochloride Mycophenolate Mofetil Salmeterol Xinafoate Indacaterol Maleate Difluprednate Fingolimod Meloxicam Amphotericin B Dulaglutide Naldemedine Secukinumab Levalbuterol Dorzolamide -

2 Total Pharmaceutical Sales
OECD Health Statistics 2021 Definitions, Sources and Methods Total pharmaceutical sales Total sales of pharmaceutical products on the domestic market, in total and by selected Anatomical Therapeutic Chemical (ATC) classification groups, based on retail prices (which means the final price paid by the customer). The ATC codes below are based on the 2021 version of the ATC Index. All alterations implemented from January 2021 are available on the WHO Collaborating Centre for Drug Statistics Methodology website at http://www.whocc.no/atc/lists_of_new_atc_ddds_and_altera/alterations_in_atc_ddd/. Note: There are at least three possible sources of under-reporting of drug sales in different countries: 1) sales data may only cover those drugs that are reimbursed by public insurance schemes; 2) they may be based on ex-factory or wholesale prices rather than retail prices; and 3) sales data may exclude drug consumption in hospitals. Data for the following countries under-estimate pharmaceutical sales reported in this section because of one of these limitations: Australia, Austria, France, Germany, Greece, Japan, Luxembourg, the Netherlands, the Slovak Republic (before 2016) and Spain. (For further information, see the country-specific information below). Please also note that depending on the allocation of pharmaceutical products with more than one use, differences in reporting of specific drugs may occur across countries, thereby affecting the relative size of specific ATC groups. Data should reflect total sales for each drug category, based on -

Staying on Schedule: How to Take Each HIV Medicine
11 1 11 12 1 11 1 11 12 1 10 2 10 2 10 2 10 2 9 33 9 33 9 33 9 33 8 4 8 4 8 4 8 4 7 6 5 7 6 5 7 6 5 7 6 5 Staying on Schedule How to take each HIV medicine HIV medicines are a key part of your HIV treatment. They can reduce the amount of HIV in your blood to very low levels and help restore your immune system health and your overall health. When you start taking HIV medicines, it is a big commitment. You have to take HIV medicines on time, exactly as they are prescribed, for them to work properly. What this booklet does: • Shows a picture of each HIV medicine. • Lists the amount of the drug in each dose (the amount you take may vary). • Tells you when to take the medicine and whether or not to eat food with it. • Gives general tips for taking each HIV medicine. An HIV medicine schedule is different for everyone. Your doctor or health care provider will work closely with you to decide which medicines to take and how much to1 take. Ask questions before you start taking a medicine When you pick up a new prescription or a refill of an HIV medicine at the drugstore, read the directions carefully. If you don’t understand anything about taking the medicine, ask the pharmacist to explain. Make sure the medicines look the same as the ones you are taking. Check to see if the instructions for taking them are the same instructions given by your doctor or health care provider. -

Geisinger Lewistown Hospital Published: March 25, 2019
Geisinger Lewistown Hospital Published: March 25, 2019 DESCRIPTION CHARGE Fine needle aspiration; without imaging guidance $ 607.00 Fine needle aspiration; without imaging guidance $ 286.00 Fine needle aspiration; with imaging guidance $ 2,218.00 Fine needle aspiration; with imaging guidance $ 1,691.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; first lesion $ 1,979.00 Placement of soft tissue localization device(s) (eg, clip, metallic pellet, wire/needle, radioactive seeds), percutaneous, including imaging guidance; each $ 1,385.00 additional lesion (List separately in addition to code for primary procedure) Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); simple or single $ 657.00 Incision and drainage of abscess (eg, carbuncle, suppurative hidradenitis, cutaneous or subcutaneous abscess, cyst, furuncle, or paronychia); complicated or $ 986.00 multiple Incision and drainage of pilonidal cyst; simple $ 657.00 Incision and drainage of pilonidal cyst; complicated $ 3,726.00 Incision and removal of foreign body, subcutaneous tissues; simple $ 1,694.00 Incision and removal of foreign body, subcutaneous tissues; complicated $ 4,710.00 Incision and drainage of hematoma, seroma or fluid collection $ 3,470.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 1,272.00 Puncture aspiration of abscess, hematoma, bulla, or cyst $ 657.00 Incision -

ENTRESTO (Sacubitril and Valsartan) Is a Combination of a Neprilysin Inhibitor and an Angiotensin II Receptor Blocker
HIGHLIGHTS OF PRESCRIBING INFORMATION • Adjust adult doses every 2 to 4 weeks and pediatric doses every 2 weeks These highlights do not include all the information needed to use to the target maintenance dose, as tolerated by the patient. (2.2, 2.3) ENTRESTO safely and effectively. See full prescribing information for • Reduce starting dose to half the usually recommended starting dosage for: ENTRESTO. – patients not currently taking an ACE inhibitor or ARB or previously ENTRESTO® (sacubitril and valsartan) tablets, for oral use taking a low dose of these agents (2.5) Initial U.S. Approval: 2015 – patients with severe renal impairment (2.6) – patients with moderate hepatic impairment (2.7) WARNING: FETAL TOXICITY See full prescribing information for complete boxed warning. ----------------------DOSAGE FORMS AND STRENGTHS-------------------- • When pregnancy is detected, discontinue ENTRESTO as soon as • Film-coated tablets: 24/26 mg; 49/51 mg; 97/103 mg (3) possible. (5.1) --------------------------------CONTRAINDICATIONS---------------------------- • Drugs that act directly on the renin-angiotensin system can cause • Hypersensitivity to any component. (4) injury and death to the developing fetus. (5.1) • History of angioedema related to previous ACEi or ARB therapy. (4) • ----------------------------RECENT MAJOR CHANGES------------------------- Concomitant use with ACE inhibitors. (4, 7.1) • • Indications and Usage, Adult Heart Failure (1.1) 2/2021 Concomitant use with aliskiren in patients with diabetes. (4, 7.1) ----------------------------INDICATIONS AND USAGE-------------------------- ------------------------WARNINGS AND PRECAUTIONS---------------------- ENTRESTO is indicated: • Observe for signs and symptoms of angioedema and hypotension. (5.2, 5.3) • to reduce the risk of cardiovascular death and hospitalization for heart • Monitor renal function and potassium in susceptible patients. -

Minutes of PRAC Meeting on 09-12 July 2018
6 September 2018 EMA/PRAC/576790/2018 Inspections, Human Medicines Pharmacovigilance and Committees Division Pharmacovigilance Risk Assessment Committee (PRAC) Minutes of the meeting on 09-12 July 2018 Chair: June Raine – Vice-Chair: Almath Spooner Health and safety information In accordance with the Agency’s health and safety policy, delegates were briefed on health, safety and emergency information and procedures prior to the start of the meeting. Disclaimers Some of the information contained in the minutes is considered commercially confidential or sensitive and therefore not disclosed. With regard to intended therapeutic indications or procedure scope listed against products, it must be noted that these may not reflect the full wording proposed by applicants and may also change during the course of the review. Additional details on some of these procedures will be published in the PRAC meeting highlights once the procedures are finalised. Of note, the minutes are a working document primarily designed for PRAC members and the work the Committee undertakes. Note on access to documents Some documents mentioned in the minutes cannot be released at present following a request for access to documents within the framework of Regulation (EC) No 1049/2001 as they are subject to on- going procedures for which a final decision has not yet been adopted. They will become public when adopted or considered public according to the principles stated in the Agency policy on access to documents (EMA/127362/2006, Rev. 1). 30 Churchill Place ● Canary Wharf ● London E14 5EU ● United Kingdom Telephone +44 (0)20 3660 6000 Facsimile +44 (0)20 3660 5555 Send a question via our website www.ema.europa.eu/contact An agency of the European Union © European Medicines Agency, 2018. -

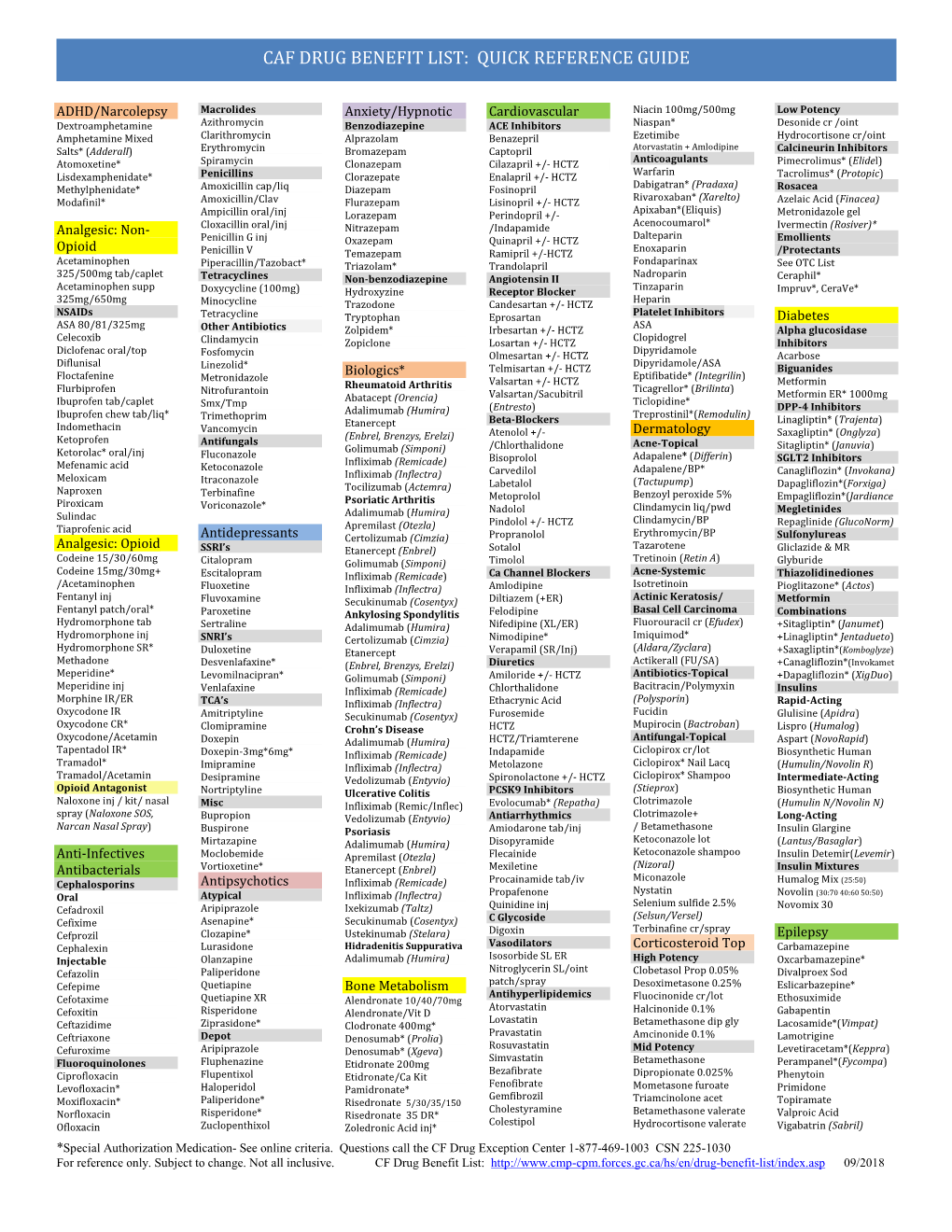
Preferred Drug List
Comprehensive PREFERRED DRUG LIST MHS Indiana Effective 04/01/2017 PAGE 1 LAST UPDATED 04/2017 Pharmacy Program MHS Health Plan (MHS) is committed to providing appropriate, high-quality, and cost- effective drug therapy to all MHS members. MHS works with providers and pharmacists to ensure that medications used to treat a variety of conditions and diseases are covered. MHS covers prescription medications and certain over-the-counter (OTC) medications when ordered by an Indiana Medicaid enrolled MHS practitioner. The pharmacy program does not cover all medications. Some medications require prior authorization (PA) or have limitations on age, dosage, and maximum quantities. For the most current information about the MHS Pharmacy Program you may call Member Services at (877) 647-4848 (TTY/TTD (800) 743-3333) or visit the MHS website www.mhsindiana.com. Preferred Drug List The MHS Preferred Drug List (PDL) is the list of covered drugs. The PDL applies to drugs that members can receive at retail pharmacies. The MHS PDL is continually evaluated by the MHS Pharmacy and Therapeutics (P&T) Committee to promote the appropriate and cost-effective use of medications. The Committee is composed of the MHS Medical Director, MHS Pharmacy Director, and several Indiana physicians, pharmacists, and specialists. Pharmacy Benefit Manager Envolve Pharmacy Solutions (EPS) is our Pharmacy Benefit Manager. MHS works with EPS to process all pharmacy claims for prescribed drugs. Some drugs on the MHS PDL require PA, and EPS is responsible for administering this process. Specialty Drugs Certain medications are only covered when supplied by MHS’ specialty pharmacy provider. AcariaHealth is our specialty pharmacy provider. -

Summary of Product Characteristics 1. Name Of
SUMMARY OF PRODUCT CHARACTERISTICS 1. NAME OF THE MEDICINAL PRODUCT Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 5 mg amlodipine. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: each capsule contains 5 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine and . Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to5 mg amlodipine. Ramipril/Amlodipine Glenmark, 10 mg/10 mg, hard capsules: each capsule contains 10 mg ramipril and amlodipine besilate equivalent to 10 mg amlodipine . For the full list of excipients, see section 6.1. 3. PHARMACEUTICAL FORM Hard capsule Ramipril/Amlodipine Glenmark, 5 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque pink colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 5 mg/10 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque red - brown colour, body: opaque white colour. Content of capsules: white or almost white powder. Ramipril/Amlodipine Glenmark, 10 mg/5 mg, hard capsules: hard gelatin capsules with a length of 19 mm, cap: opaque dark pink colour, body: opaque white colour. Content of capsules: white or almost white powder. -

MSM Chapter 1200 3/1/21
MEDICAID SERVICES MANUAL TRANSMITTAL LETTER February 23, 2021 TO: CUSTODIANS OF MEDICAID SERVICES MANUAL FROM: JESSICA KEMMERER, HIPAA PRIVACY AND CIVIL RIGHTS OFFICER /Jessica Kemmerer/ BACKGROUND AND EXPLANATION The DHCFP is proposing revisions to Medicaid Services Manual (MSM), Chapter 1200 – Prescribed Drugs, Appendix A, to reflect recommendations approved on October 22, 2020, by the Drug Use Review (DUR) Board. The proposed changes include the addition of new prior authorization criteria for Doxepine Topical, the addition of new prior authorization criteria for Zeposia® (ozanimod), addition of new prior authorization for Evenity® (romosozumab-aqqg), Prolia® (denosumab), Forteo® (teriparatide) and Tymlos® (abaloparatide) within a new combined osteoporosis agents section, and addition of new prior authorization criteria for Orilissa® (elagolix) and Oriahnn® (elagolix, estradiol, and norethindrone) within a new Gonadorpin Hormone Receptor (GnRH) Antagonist and Combinations section. Additionally, the DHCFP is proposing revisions to the existing prior authorization criteria for psychotropic medications for children and adolescents, and revision to the existing clinical criteria for Epidiolex® (cannabidiol). Throughout the chapter, grammar, punctuation and capitalization changes were made, duplications removed, acronyms used and standardized, and language reworded for clarity. Renumbering and re- arranging of sections was necessary. These changes are effective March 1, 2021. MATERIAL TRANSMITTED MATERIAL SUPERSEDED MTL N/A MTL N/A MSM Ch 1200 – Prescribed Drugs MSM Ch 1200 – Prescribed Drugs Background and Explanation of Policy Changes, Manual Section Section Title Clarifications and Updates Appendix A Psychotropic Added new policy language criteria on which specific Section N Medications for drug classes may bypass polypharmacy clinical criteria. Children and Adolescents Appendix A Reserved for Future Created a new section titled “Doxepin Topical.” Added Section W Use new prior authorization criteria for doxepin topical. -

Glaxosmithkline Plc Annual Report for the Year Ended 31St December 2000
GlaxoSmithKline 01 GlaxoSmithKline plc Annual Report for the year ended 31st December 2000 Contents Report of the Directors 02 Financial summary 03 Joint statement by the Chairman and the Chief Executive Officer 05 Description of business 29 Corporate governance 37 Remuneration report 47 Operating and financial review and prospects 69 Financial statements 70 Directors’ statements of responsibility 71 Report by the auditors 72 Consolidated statement of profit and loss 72 Consolidated statement of total recognised gains and losses 74 Consolidated statement of cash flow 76 Consolidated balance sheet 76 Reconciliation of movements in equity shareholders’ funds 77 Company balance sheet 78 Notes to the financial statements 136 Group companies 142 Principal financial statements in US$ 144 Financial record 153 Investor information 154 Shareholder return 156 Taxation information for shareholders 157 Shareholder information 158 Share capital 160 Cross reference to Form 20-F 162 Glossary of terms The Annual Report was approved by the Board 163 Index of Directors on 22nd March 2001 and published on 12th April 2001. Contact details 02 GlaxoSmithKline Financial summary 2000 1999 Increase Business performance £m £m CER % £ % Sales 18,079 16,164 9 12 Trading profit 5,026 4,378 12 15 Profit before taxation 5,327 4,708 11 13 Earnings/Net income 3,697 3,222 13 15 Earnings per Ordinary Share 61.0p 52.7p 14 16 Total results Profit before taxation 6,029 4,236 Earnings/Net income 4,154 2,859 Earnings per Ordinary Share 68.5p 46.7p Business performance: results exclude merger items and restructuring costs; 1999 sales and trading profit exclude the Healthcare Services businesses which were disposed of in 1999. -
Clinical Teach Back Cards
Medication Safety Clinical Teach-Back Cards* The Medicare Quality Innovation Network Quality Improvement Organization (QIN-QIO) for Texas, Missouri, Oklahoma, Arkansas and Puerto Rico TMF Health Quality Institute focuses on improving lives by improving the quality of health care through contracts with federal, state and local governments, as well as private organizations. For nearly 40 years, TMF has helped health care providers and practitioners in a variety of settings improve care for their patients. *Content subject to change Medication Safety Clinical Teach-Back Cards TMF Health Quality Institute Bridgepoint I, Suite 300 5918 West Courtyard Drive Austin, TX 78730-5036 1-866-439-6863 Phone: 512-329-6610 Fax: 512-334-1775 www.tmfqin.org This material was developed by TMF Health Quality Institute, the Medicare Quality Innovation Network Quality Improvement Organization under contract with the Centers for Medicare & Medicaid Services (CMS), an agency of the U.S. Department of Health and Human Services. This content does not necessarily reflect CMS policy. 11SOW-QINQIO-C3.6-16-01 TEACH-BACK “I want to make sure I explained this clearly. When you get back home in a few days, what will you tell your [friend or family member] about [key point just discussed]?” Do NOT ask the patient, “Do you understand?” TEACH-BACK The “teach-back” technique is an effective method for ensuring that patients understand what you have told them. It involves asking patients to explain or demonstrate what they have been told. For example, you can say, “Please show me how you will use the asthma inhaler, so I can be sure I have given you clear instructions.” ANGIOTENSIN- CONVERTING ENZYME (ACE) INHIBITORS These drugs improve symptoms and prevent symptoms from worsening by relaxing blood vessels, controlling fluid and slowing the progression of heart failure. -

Drugs Requiring Preauthorization
DRUGS REQUIRING PREAUTHORIZATION The table below outlines the medications requiring a review by the Clinical Pharmacist, and if necessary, a Health Alliance Medical Director. If a provider wished for coverage of a drug designated as preauthorization required (PA), they must provide documentation to meet criteria for that particular medication. Provider must request prior authorization from Health Alliance for drugs on the following list: Drug Class Drug Name Comments ASTHMA/ COPD Advair® (fluticasone-salmeterol) See Non-Preferred ICS/LABA Combination Breo™ Ellipta® (fluticasone-vilanterol) Inhalers policy Arnuity™ Ellipta® (fluticasone- See Non-Preferred ICS Inhalers policy salmeterol) ArmonAir™ RespiClick® (fluticasone propionate) Daliresp® (roflumilast) See Daliresp policy BEHAVIORAL Dyanavel™ XR (amphetamine Member aged 6 to 12; documentation of HEALTH: suspension) inability to swallow tablets for members ADHD Quillichew® ER (methylphenidate ER) older than 12 Quillivant XR® (methylphenidate suspension) Vyvanse® chewable (lisdexamfetamine) BEHAVIORAL desvenlafaxine ER See Behavioral Health policy; HEALTH: Fetzima™ (levomilnacipran ER) two Tier 1 SSRIs and two Tier 1 SNRIs Antidepressants Khedezla™ (desvenlafaxine ER) (duloxetine and venlafaxine/venlafaxine ER) Pristiq® (desvenlafaxine) Trintellix® (vortioxetine) Viibryd® (vilazodone) BEHAVIORAL aripiprazole See Behavioral Health policy; HEALTH: Rexulti® (brexpiprazole) as adjunct therapy for Major Depressive Atypical Disorder: TWO Tier 1 SSRIs, AND TWO Tier Antipsychotics 1 SNRIs; for